Purification and Characterization of Isocitrate Dehydrogenase from Chlamydomonas reinhardtii
Edward Gordon, Chad Newman, A. Malcolm Campbell, and John H. Williamson
Biology Department, Davidson College
ABSTRACT
NADP-dependent isocitrate dehydrogenase was
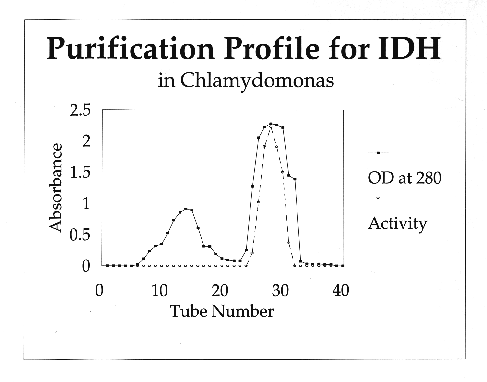
purified from Chlamydomonas reinhardtii using ammonium sulfate precipitation,
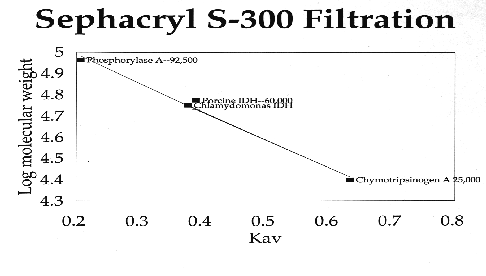
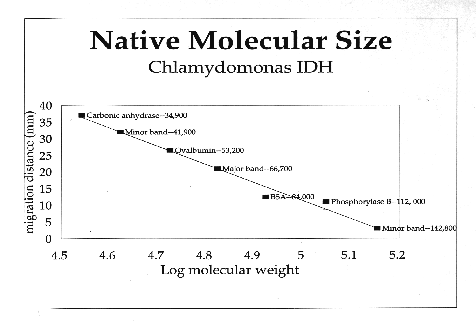
affinity chromatography and gel filtration.
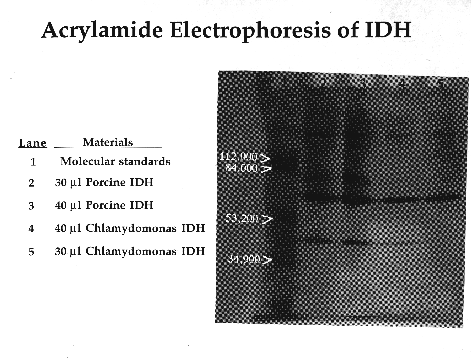
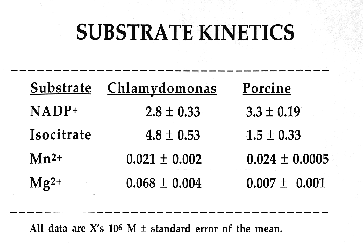
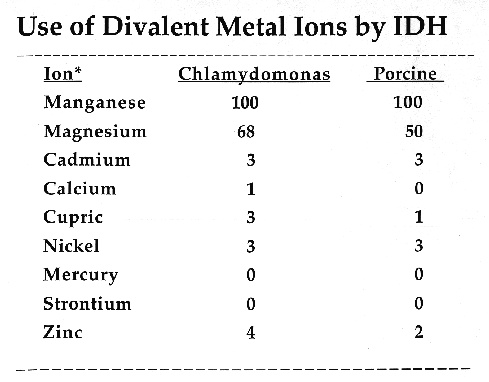
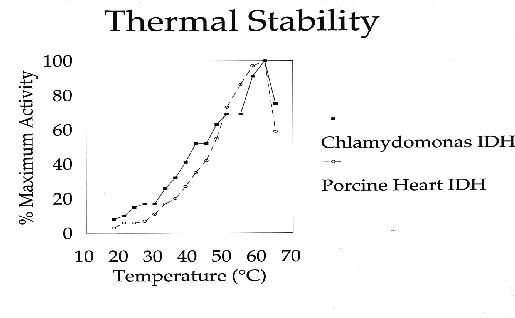
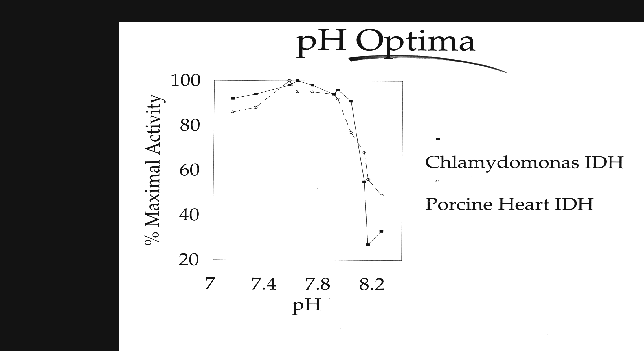
C. reinhardtii IDH is a monomeric enzyme with a molecular size of 60 kD. Km values for isocitrate and NADP were 4.8 ±0.53 µM and 2.8 ±0.33 µM, respectively. The enzyme has an absolute requirement for divalent metal ions, using Mn++ about 1.5 times as efficiently as Mg++, but cannot use Cd, Ca, Cu, Ni, Hg, Sr or Zn ions. IDH activity is stable to about 60·C and has a wide pH optimum of 7.4 to 8.2.

© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to: jowilliamson@davidson.edu