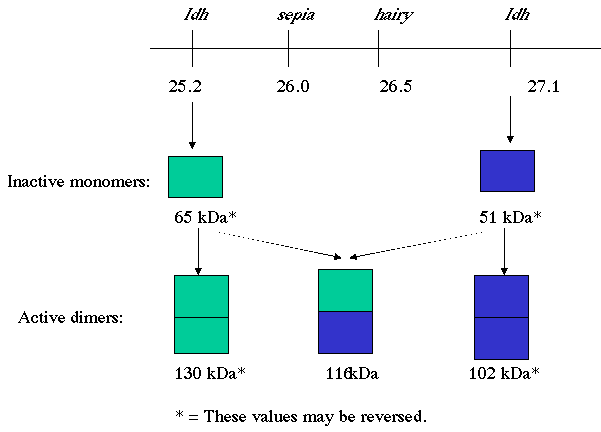
In combination with the reports of two Idh loci, our data on the molecular size of IDH and its subunits has led to the construction of a novel model to explain the genetic regulation of IDH activity in Drosophila melanogaster (Figure 8). There are two closely linked Idh loci, one on either side of the hairy locus (Figure 2; Lindsley and Zimm, 1992). Each locus encodes a subunit of IDH and the two subunits are different in size. These subunits combine randomly and all combinations of these two subunits yield an active dimer of IDH. Therefore, three active forms of IDH, of different sizes, are possible. Furthermore, the frequencies of these active forms would be predicted to be found in a 1 : 2 : 1 ratio of homodimers of the smaller subunit: heterodimers of both subunits: homodimers of the larger subunit. When non-denaturing gel electrophoresis was conducted and the gel stained for IDH activity, three bands would appear, much as we observed (Figure 6A). SDS-PAGE would separate the two subunits of the three classes of IDH and two protein bands of different molecular sizes would be observed. Our results are consistent with such a prediction (Figure 7). The ultimate test of our model will become possible when the Drosophila Genome Project is complete.
