Transcytosis
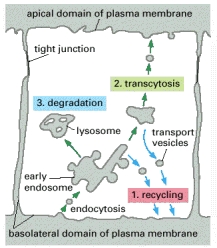
Transcytosis occurs as membrane-bound carriers selectively transport materials between one part of the cell and another in order to maintain unique environments on either side of the cell. Epithelial cells use transcytosis for immune defense, nutrient absorption, and plasma membrane biogenesis. Other cell types employ transcytosis as well, such as the endothelium and the endocrine system.
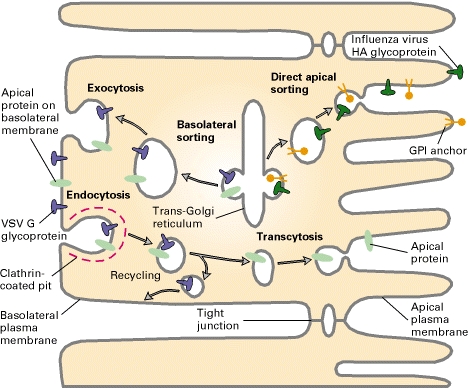
Figure 1. Mechanisms of transport within a cell. Transcytosis, in red font, is shown as cargo is transported from one side of the cell to the other. CME signifies clathrin-mediated endocytosis and CAV represents caveolae mediated.
Source: Baluska, Frantisek, Dieter Volkmann, and Peter W. Barlow. "Channels across Endothelial Cells." Cell-cell Channels. Georgetown (Tex.): Landes Bioscience, 2006. Permission pending.
Two types of transcytosis exist, differing in mechanisms of vesicle formation and major proteins. Their descriptions are seen below.
Caveolae-Mediated Transcytosis |
Clathrin-Mediated Transcytosis |
|
|
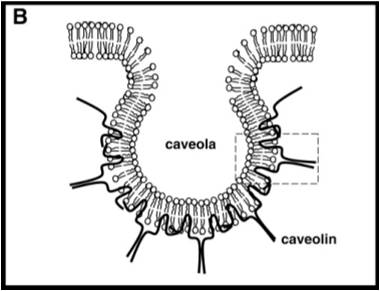
Endothelial cells, specialized epithelium that line blood vessels, utilize caveolae-mediated transcytosis. Caveolae are pits in the apical and basal membranes of all endothelial cells, named for their “small cave” shape. The major structural component of caveolae, shown in Figure 1, is caveolin. These vesicles transport cargo, usually fluid, from the apical to basal or basal to apical surfaces of the cells. The caveolae can merge to create arrangements shown above (Figure 3), including a tunnel or channel, in order to move cargo to other side of cell. |
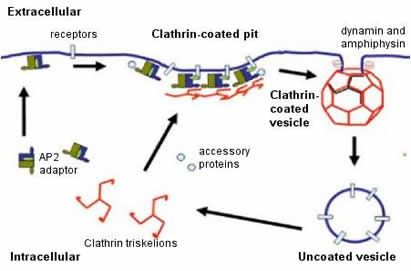
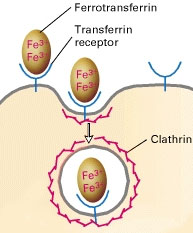
Transcytosis is used extensively by epithelial cells as a part of the immune response. Clathrin, a protein located on both apical and basal surfaces of the epithelial cells, lines these vesicles. Clathrin-mediated transcytosis is a way for these cells to sort through the “cargo” of molecules entering the cell as one of the destinations of these vesicles is the Golgi. On the surface of the cell membrane, a “pit” forms from specific cell receptors that are coated by clathrin. The protein clathrin’s purpose is to stabilize the forming vesicle after the receptors have bound and begin to invaginate. Clathrin achieves this by forming a rigid matrix of an assembling of clathrin proteins, which can later disassemble after the vesicle has disassociated from the cell membrane. Vesicles attach to the endoplasmic reticulum before being "sorted" to either the apical or basal side of the cell. See Figure 1. |
Purpose and Locations In the Body
| Vascular System: The vascular system uses transcytosis to manage concentrations of many required molecules in the blood and the surrounding tissue. Caveolae-mediated transport via localized receptors in the plasma membrane of the endothelial cells determine the movement of these macromolecules. |
|
|
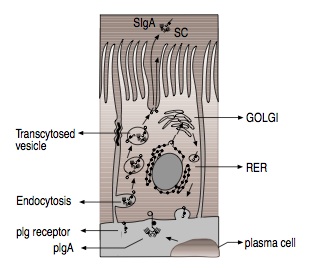
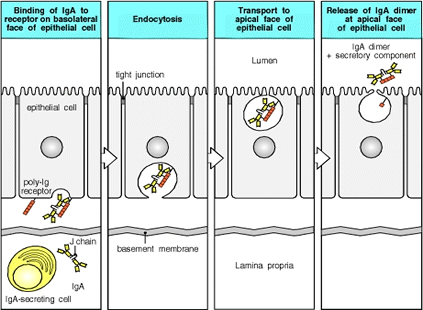
Immune System: As the body’s initial location for exposure to environmental hazards, such as viruses or bacteria, the epithelial cells must bind to secreted immunoglobulin proteins as a defense mechanism. Transcytosis by clathrin-mediated mechanisms is used for this purpose, as IgA, an immunoglobulin, is moved from the basal to apical membranes of the digestive tract epithelium (Figure 6). |
| Micronutrients: Most micronutrients do not use vesicle-mediated means to reach the blood stream. Vitamin B12 and iron are exceptions in that they use transcytosis. Both are essential factors in homeostasis. Iron is taken up in the form of transferrin from the digestive tract. Iron binds to transferrin where it is endocytosed through clathrin-mediated transcytosis. Once endocytosed, the transferrrin receptor frees itself from the vesicle and is recycled back on the apical surface. Iron is then expelled on the basolateral surface via endocytosis and into the bloodstream. B12 is also protein-mediated in a similar fashion and found in digested meat in the digestive tract. |
|
|
Plasma Membrane Biogenesis: Most cargo of transcytosis is sent outside the cell. However, in some locations in the body, specifically the hepatocyte and the enteroctye of the intestinal villus, transcytosis is used to transport the cell’s newly synthesized proteins to their correct locations within the plasma membrane. Hepatocytes and enterocytes typically endocytose proteins from the basolateral membrane surface (usually the surface exposed to blood), these proteins can then be transported by endosomes to the apical surface where the fuse with the apical membrance. The vice-versa can also occur with protein translocation from the apical surface to the basolateral surface. Another mechanism in mediating protein movement to the polarized membrane surfaces is transcytosis via Trans-Golgi reticulum as a mediator, using vesicles and assistance of transport proteins (SNARE, etc.). This is one way to maintain the polarity of epithelial cells. |
Additional Organ Systems:
|
|
While transcytosis is a process that helps define epithelial cells, these cells also use additional methods of cellular transport including diffusion, osmosis, active transport, endocytosis and exocytosis.
Research continues in the following areas:
- Specific mechanisms of caveolae-mediated transcytosis
- Role of transcytosis in development
- Connections between dysfunction of transcytosis and disease
- Cell signaling for degradation versus transcytosis
- Role of lysosomes within transcytosis
Alberts, Bruce. "Intracellular Vesicular Traffic." Molecular Biology of the Cell. New York: Garland Science, 2002.
Baluska, Frantisek, Dieter Volkmann, and Peter W. Barlow. "Channels across Endothelial Cells." Cell-cell Channels. Georgetown (Tex.): Landes Bioscience, 2006.
Berk, Arnold, Lawrence Zipursky, Paul Matsudaira, and David Baltimore. "Protein Sorting: Organelle Biogenesis and Protein Secretion." Molecular Cell Biology. By Harvey Lodish. New York: W.H. Freeman, 2000.
Durbin, R. P., & Hill, A. (1981). Osmosis in epithelial membranes. Journal of Membrane Biology, 61(2), 141-142. doi:10.1007/BF02007641.
Janeway, C. (2001). Immunobiology: The immune system in health and disease . New York: Garland.
Lodish, H., Berk, A., Kaiser, C. A., Krieger, M., Scott, M. P., Bretscher, A., Ploegh, H., & Matsudaira, P. (2008). Molecular cell biology (6th ed.) W.H. Freeman.
Miot, F., Dupuy, C., Dumont, J. E. & Rousset, B. A. (2010). Thyroid hormone synthesis and secretion. Retrieved 10/3, 2010, from http://www.thyroidmanager.org/Chapter2/2-frame.htm
Overview of transport across the intestinal epithelium Retrieved 10/3/2010, 2010, from http://www.vivo.colostate.edu/hbooks/pathphys/digestion/smallgut/transport.html
Pilette, C., Ouadrhiri, V., Godding, J. P., & Sibille, Y. (2001). Lung mucosal immunity: Immunoglobulin-A revisited. European Respiratory Journal, 18, 571.
Tuma, P. L., & Hubbard, A. L. (2003). Transcytosis: Crossing cellular barriers. Physiology Reviews, 83, 871.
Wu, X., Zhao, X., Baylor, L., Kaushal, S., Eisenberg, E., & Greene, L. E. (2001). Clathrin exchange during clathrin-mediated endocytosis. The Journal of Cell Biology, 155(2), pp. 291-300.