Fall 1999 Biology 111 Exam #1 - Cellular Communications
There is no time limit on this test, though I have tried to design one that you should be able to complete within 2.5 hours, except for typing. You are not allowed to use your notes, old tests, the internet, or any books, nor are you allowed to discuss the test with anyone until all exams are turned in at 11:30 am on Monday September 20. EXAMS ARE DUE AT CLASS TIME ON MONDAY SEPTEMBER 20. You may use a calculator and/or ruler. The answers to the questions must be typed on a separate sheet of paper unless the question specifically says to write the answer in the space provided. If you do not write your answers in the appropriate location, I may not find them.
Please do not write or type your name on any page other than this cover page.
Staple all your pages (INCLUDING THE TEST PAGES) together when finished with the exam.
Name (please print):
Write out the full pledge and sign:
Here is the honor code
http://www.davidson.edu/student/redbook/honorgeneral.html#honorcode
"On my honor I have neither given nor received unauthorized information regarding this work, I have followed and will continue to observe all regulations regarding it, and I am unaware of any violation of the Honor Code by others."
How long did this exam take you to complete (excluding typing)?
Lab Questions:
4 pts.
1) I have to make a confession. The neutral red solution you used
in lab the first week was not 1M but in fact it was 0.1% w/v.
Furthermore, the MW for neutral red is not 100, it is really 87.5.
a) Tell me how we made 450 mL of this 0.1% w/v solution.
Dissolve 0.45g in 400 mL, then bring up
to 450 mL with water.
b) What is the molarity of the solution you made in the test
tube during lab that was 4% v/v of the stock solution which we
told you was 1M but now you know is 0.1%solution? So calculate
the true molarity of your "4% v/v" solution.
0.00044M or 0.44 mM or 440 µM
6 pts.
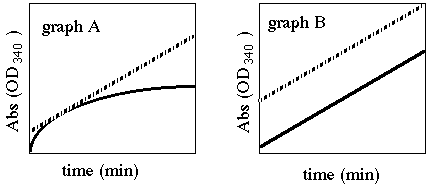
2) There are two parts to this question, A and B, which go
with the two graphs below. Tell me in "plain English"
what experimental conditions could alter an enzyme's reaction
such that when graphed, it looks like the solid lines below, compared
to the dashed lines from an ideal experiment? For both A and B,
explain why this altered condition would produce its new graph.
Graphs A and B represent different parts to this question and
they should be answered separately.
A In this reaction, there does not appear
to be enough substrate so the rate of the reaction starts off
fast, but slows as the substrate is consumed. Therefore the graph
levels off as no new product is made to increase the amount of
light absorbed.
B In this graph, since the slopes appear to be identical, there appears to be no change in the reaction conditions. The only difference is the Y intercept which can be affected by changing the amount of time between when the reaction was started and when it was read by the machine.

Lecture Questions:
6 pts.
3) A major tenet in biology is that form follows function.
Give an example that illustrates this point on the cellular level.
Explain how this example illustrates the point.
The epinephrine receptor has a shape that
allows it to bind epinephrine but not other compounds. When epi
binds, it changes the shape of the receptor which enables it to
activate G proteins and initiate a cascade of enzymes.
This illustrates the point because the form (or shape) allows
it to bind epi and then change its shape to change its function;
now it can activate G proteins. Its function is determined by
its shape.
6 pts.
4) What are the chemical differences between starch, glycogen,
and cellulose? What significance do these differences have in
your daily life?
.Starch and glycogen are very similar, but
they do have slight differences in their banching of sugars, but
both use a1-4 glycosidic linkage to connect two glucose
molecules. Cellulose is very different polymer of glucose since
the monomers are linked via b1-4 glycosidic linkages. The significance is
that humans cannot hydrolyze b1-4 linkages but we can hydrolyze a1-4
linkages. Therefore, we can extract sugars from starch and glycogen,
but not cellulose.
8 pts.
5) Explain how changing one amino acid in a G protein could change
its overall function. In your answer, properly use the different
terms that describe the levels of protein structure with regards
to G proteins.
If a critical amino acid is changed from
hydrophobic to positively charged for example, then all the neighboring
amino acids will repsond due to the interactions of all these
R groups. Since the primary amino acid sequence determines the
secondary structure, this alteration might disrupt an alpha helix
or beta pleated sheet. Furthermore, the amino acid R groups also
determine tertiary structure and if this amino acid were involved
in bonds that held the tertiary structure, then the protein would
have the wrong shape and thus not be able to function properly.
Finally, G proteins have quaternary structure, there are 3 subunits.
If the altered amino acid affected the way the 3 subunits fit,
then the G protein could not function properly, again.
If the amino acid in question was involved in the hydrolysis of
GTP to GDP, then this G protein would never be deactivated. Or
if this amino acid was necessary for the binding of GTP, then
this mutant G protein could never be activated if it never bound
GTP.
5 pts.
6) An international team has found estrogen can bind to two
different receptors, called alpha and beta. When estrogen binds
to its alpha receptor, genes are activated, while binding to the
beta receptor inhibits gene activation. Hypothesize a molecular
mechanism to explain how the same ligand could give two different
signals.
.Receptors have two business ends. One end
binds the ligand, the other binds to the internal protein that
initiates the cascade. If the ligand binding portion were the
same for these two receptors but their intracellular portions
were different, then they could both bind estrogen but initiate
different consequences inside their cells by activating different
intracellular proteins.
10 pts.
7) a) What category of modulation is needed to activate
glycogen phosphorylase and glycogen synthase?
Both require covalent modulation to be activated.
b) Describe what a cell must do to activate each of
these two enzymes.
A cell must phosphorylate glycogen phosphorylase
and dephosphorylation glycogen synthase.
c) Which event, or events, of your answers from part b above
happens when you get scared?
A liver cell phosphorylates glycogen phosphorylase.
d) What does the enzyme phosphorylase kinase do in your liver
and how is it activated?
Phosphorylase kinase phosphorylates glycogen
phosphorylase. Phosphorylase kinase is activated when it is phosphorylated
by protein kinase-A.
e) What does the enzyme protein kinase A do in your liver and
how is it activated?
Protein kinase A phosphorylates phosphorylase
kinase to activate the latter. Protein kinase A is activated by
the binding of cAMP.
6 pts.
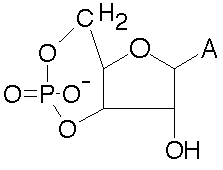
8) In the space below, draw a picture of cAMP. In your drawing,
you do not have to draw the base, simply write the letter that
represents the base.
5 pts.
9) Tell me exactly what role ATP plays in pumping calcium
ions across the SER membrane. I want you to be very specific and
not say something general like "it provides energy."
Tell me exactly which steps in the pumping cycle it facilitates
and how.
ATP is an allosteric modulator of the calcium
pump so that when it binds to the pump, the pump takes the terminal
phosphate from ATP and uses it to phosphorylate itself. When the
pump gets phosphorylated, this covalent modulation makes the pump
flip. Flipping the pump flips the two bound calcium ions from
the cytoplasmic side to the lumenal side of the membrane. Once
the calcium pump flips, it no longer holds onto the calcium with
high affinity and so the calcium ions float away inside the SER.
When the ions float away, the phosphate's covalent bond is broken
and the phosphate floats away. When the phosphate bond is broken,
the pump flips back to reveal the calcium binding sites on the
cytoplasmic side again. All of these changes are cuased by R groups
adjusting to the changes in charges experienced when the ATP binds
and when the phosphate is covalently bound.
15 pts.
10) Create a table with 3 columns in it and many rows (this
can be a hand-drawn table if you don't know how to create a table
with your word processor). In this table, list as many examples
as you can think of that we have studied so far. For each
row provide the following information:
Column A: a mechanism where calcium ions were used in intracellular communication
Column B: where the calcium ions came from for each example in column A
Column C: What protein allowed it to cross a membrane and go down its concentration gradient for each example in column A and B.
|
Column A |
Column B |
Column C |
| exocytosis in neuron | outside neuron | voltage-gated Ca2+ channel |
|
heart muscle contraction skeletal muscle contraction |
outside (heart) and SR (skeletal muscle) |
voltage-gated Ca2+ channel (heart) unknown-gated Ca2+ channel (skeltal) |
|
Egg block to poly spermy (exocytosis again) and activation |
came from ER came from ER |
IP3 receptor Ca2+-gated Ca2+ channel (CICR) |
6 pts.
11) How is resting membrane potential created? In your answer,
include the structure of the plasma membrane, the protein responsible
for generating the potential, and the ion or ions involved.
The plasma membrane is a phospholipid bilayer
which means that charged particles cannot cross it without the
assistance of a protein.
The protein responsible for generating the resting membrane potential
is the sodium/potassium pump.
This pump transports 3 sodium ions out of the cytoplasm and 2
potassium ions in. This results in a net loss of positive charge
inside which creates a separation of charges across the membrane.
As a result, the inside is more negative by about 60-70 mV.
8 pts.
12) What happens at a presynaptic terminus that allows the
neurotransmitter to be secreted? Start your explanation AFTER
the action potential has reached the terminus.
1) The voltage-gated calcium channels open
and calcium floods the cytoplasm.
2) Calcium binds to synaptotagmin which along with VAMP is an
integral membrane protein in the secretory vesicles.
3) Synaptotagmin changes its shape in response to the Ca2+ and allosterically
causes VAMP to change its shape.
4) VAMP can now bind to syntaxin which was sitting in the plasma
membrane.
5) The secretory vesicle fuses with the plasma membrane and the
contents of neurotransmitter (acetylcholine) are dumped into the
synaptic cleft.
6 pts.
13) How can the fertilization signal be deactivated within
the egg? To receive full credit for this question, you must describe
three different mechanisms for deactivation.
Since the receptor for mystery protein X
is no longer stimulated, it stops activating G proteins.
G protein hydrolyze GTP to GDP and become inactive.
IP3 is
broken down into IP2 and can no longer bind to the IP3 receptor.
Calcium is pumped back into the ER.
4 pts.
14) As explained in one of the study questions, many cancer
researchers study signal transduction. G proteins are very important
in many cancers because mutated G proteins cannot hydrolyze GTP.
a) Explain to me in "plain English" what the phrase
"hydrolyze GTP" means.
This means that the covalent bond holding
terminal phosphate of GTP on to the rest of the molecule is broken.
A free phosphate and GDP are the products.
b) Explain why this inability to hydrolyze GTP might lead to
cancer.
Since G proteins inactivate themselves by
converting GTP to GDP, this allele of G protein cannot be inactivated
and so it continues to stimulate the cell. In this case, the stimulation
results in cell division and cancer.
5 pts.
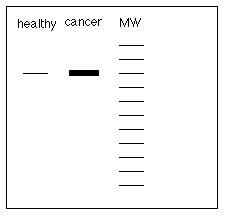
15) Some forms of breast cancer are stimulated by estrogen.
Design an experiment to determine if these breast cancer cells
have more estrogen receptors or fewer estrogen receptors than
healthy breast cells.
I would use gel electrophoresis and a western
or immunoblot. I would take biopsies of both tissues and electrophores
the proteins from both in separate lanes of the same gel. I would
purchase or make a monoclonal antibody against human estrogen
receptors. I would use it in an immunoblot and see if there is
more receptor in the cancer cells than in the healthy cells. I
would expect to see something like this if there are more receptors
in cancerous cells.
See Copy of Test with Answers
Return to Bio111 Home Page