
Lifecycle
Like other mycobacteria (or ‘acid-fast’ bacteria), Mycobacterium leprae has a difficult time replicating outside of host cells. While some researchers maintain that it is a facultative intracellular parasite, others say that the bacteria cannot replicate at all outside of the cell. The former are somewhat supported by the fact that M. leprae has never been cultured in vitro(1). When M. leprae can find a host, it is a very slowly replicating bacteria that can take up to 13 days to undergo one replication cycle. Leprosy is characterized by bacterial replication inside intracellular vesicles of macrophages, Schwann cells, and endothelial cells. In general, M. leprae prefers such cells at lower temperatures than that of the human body, which is why it tends to manifest itself near the skin’s surface.
Cell binding:
First, the bacteria binds to receptors on the host cell surface. For neural Schwann cells, the phenolic glycolipid-1 (PGL-1) or LBP21 receptor on M. leprae binds to the α-2 side chain of laminin-2 as well as the related α-dystroglycan receptor(1). However, laminin is also found on straited muscle cells and placental tissue, both of which are also infected by M. leprae (1). The presence of the histone-like protein Hlp, secreted by M. leprae, enhances Schwann cell binding (1).
PGL-1/LBP21 – laminin binding is explained more in the picture below. The laminin protein is made up of two subunits, α-dystroglycan and β-dystroglycan (2). The α subunit is located on the extracellular side of the membrane and binds three proteins: laminin- α 1 chain, agrin, and perlecan (2). This whole complex is thought to structurally stabilize the cell and protect it from injury (2).
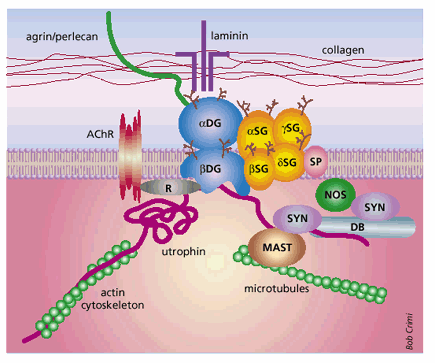
Notice the complex binding pattern of PGL-1/LBP21 to the laminin group of proteins (2- Barker 2006)
Fibronectin, βintegrin6, and a 25kDa glycoprotein also bind to M. leprae, which are present in epithelial cells (5). Studies have shown that multiple binding sites can be used by M. leprae to bind to various cell types (including endothelial cells and macrophages), indicating that if one receptor is absent, others can work just as well (1).
For macrophages, the terminal trisaccharide on the PGL-1 peptide on M. leprae binds to the complement receptors CR1, and 4, and parts of C3, which then facilitates phagocytosis by the classical complement pathway (6). However, the fatty acid side-chains must also be present on the PGL-1 molecule for binding to occur (6). Because of the specific binding to the C3 complement, phagocytosis of M. leprae into macrophages is not associated with any oxidative burst (which is generally present when bacteria are phagocytosed by monocytes)(6). The oxidative burst is usually a signal of the bacteria's destruction - therefore, the lack of this oxidative burst may be the sign that M. leprae is somehow able to evade the initial cellular response to pathogens (6).
Once binding has occurred, M. leprae is taken into the host cell by phagocytosis and is encapsulated by a phagosome. From there, the bacteria must survive phagosome-lysosome fusion and live long enough to replicate and get back out of the cell.
Bacterial Replication:
Once the bacteria has attached to a receptor on the cell surface, it is phagocytosed by the cell. The phagocytic process is not unlike other bacterial phagocytic events, in that the actin-dependent proteins tyrosine kinase, calcium-dependent protein kinase and phosphatidylinositol 3-kinase mediate the event (1). Usually, after phagocytosis, bacteria are killed through fusion with a phagolysosome and digestion by proteases and oxidizing chemicals. However, some researchers think that M. leprae is somehow able to arrest the lysosome fusion process for anywhere from 1 to 4 hours and replicate inside the phagosome instead (3, 4). As of yet, the specific mechanisms preventing fusion have not been characterized (4). By way of this mysterious process the bacteria is able to create a region of safety directly surrounding itself, called the ‘electron transparent zone’, for its transparency under electron imaging (3).
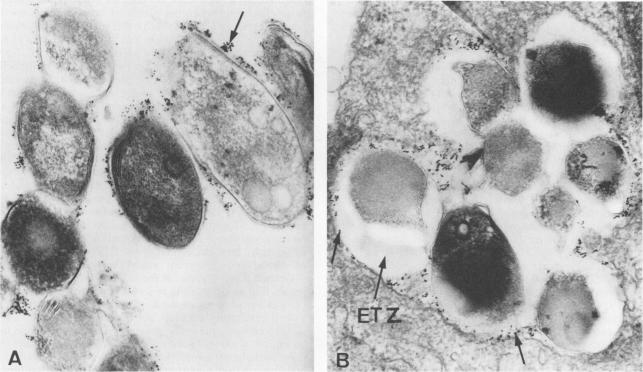
In picture B, you can clearly see the white zone around the lysosomes of macrophages infected with M. leprae. One question researchers are still researching is how long these ETZ zones persist before bacteria either replicate and escape or are destroyed by the cell (3- Frehel 1987)
However, after a certain period of hours (or if the macrophage is activated), the macrophage or Schwann cell then introduces proteases directly into the phagosome, in addition to a large number of MHC molecules (7). This second cellular response represents the first line of defense against M. leprae (7). Once the bacteria has been broken up into pieces, the MHC molecules can then take up pieces of it and present it to any passing lymphocytes or other mononuclear cells by moving to the membrane of the cell (7). The specifics of bacterial degradation and replication are not fully understood, and are still the subject of much controversy (3,4,7). If the bacteria is able to evade the degradation mechanisms and replicate inside the cell, M. leprae forms a bundle of bacteria that then break out of the cell and infect other tissue. One interesting note is that Mycobacterium leprae has never been cultured in vitro, adding to the dearth of information concerning the specific actions the bacteria takes (1).
An interesting aspect of M. leprae's lifecycle is the effect it has on the cells that it invades. Some initial studies have shown that M. leprae has the ability to enhance division of the Schwann cells it infects, therefore allowing further proliferation within the host (7). This is an interesting mechanism that isn't fully understood, but represents a novel self-protective mechanism for M. leprae.
Sources:
1. Barker, L. 2006. Mycobacterium leprae interactions with the host cell: recent advances (Review article). Indian J Med Res, 123: 748-59.
2. Chamberlain, J. 1999. The dynamics of dystroglycan. Nature Genetics, 23:256-58.
3. Frehel, C., and Rastogi, N. 1987. Phagosome-Lysosome Fusion Inhibition Event. Infect Immun, 55.12: 2916-21.
4. Jameway, C. A., Travers, P., Walport, M., and Shlomchik, M. J. Immuno Biology: the immune system in health and disease. 6th Ed. Garland Science Publishing, New York:2005.
5. Lavanya, M., Deena, V., Sujai, S., Balasubramanian, A., et al. 2001. Biochemical aspects of mycobacterium leprae binding proteins: A review of their role in pathogenesis. Int. J Leprosy and Other Mycobacterial Dis. Accessed online 1/30/2007. link
6. Schlesinger, L. S., and Horwitz, M. A. Phenolic Glycolipid-1 of Mycobacterium leprae binds complement component of C3 in Serum and Mediates Phagocytosis by Human Monocytes. J Exp Med, 174: 1031-38.
7. Steinhoff, U., Golecki, J. R., Kazda, J., and Kaufmann, S. H. E. 1989. Evidence for Phagosome-Lysosome Fusion in Mycobacterium leprae-Infected Murine Schwann Cells. Infect Imm, 57.3:1008-1010.
Copyright Alex Greer 2007
For questions/comments, please contact Immunology professor Dr. Sofia Sarafova at Davidson College.