This web page was produced as an assignment for an undergraduate course at Davidson College.
Life Cycle
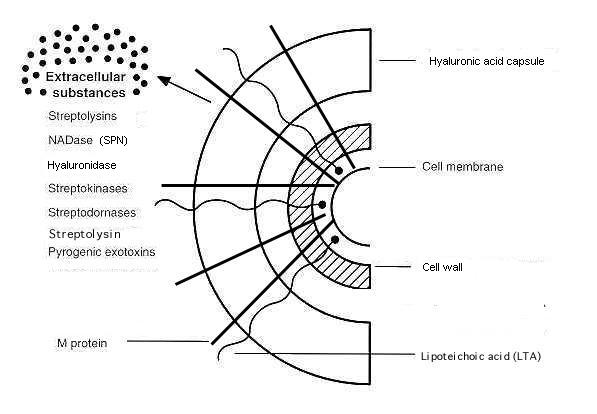
S. pyogenes is able to colonize and infect hosts via two primary methods: adhesion to host cells and the more recently discovered phenomenon of invasion of certain kinds of host cells. Three major types of molecules are used for the adhesion process: lipoteichoic acids (LTA), M protein, and fibronectin-binding proteins. LTA provides a weak adhesion to epithelial cells (again, usually in mucosal membranes), and M protein and fibronectin-binding proteins provide more secure connections. Streptococci can express several fibronectin-binding proteins, such as Protein F and Sfb, the first fibronectin-binding protein discovered in streptococci. GAS also has the ability to destroy connective tissue by secreting hyaluronidase and streptokinases, killing surrounding cells (Todar 2002).
In 1994, LaPenta et al. showed that S. pyogenes had the ability to invade cultured human epithelial cells. Prior to the publication of their article, it was known that other types of bacteria, such as Listeria, Shigella, Salmonella, and Yersinia could invade epithilial cells. The bacteria were found internalized in vacuoles in the epithelial cells. The authors also showed that invasion protected the bacteria from treatments with penicillin and gentamicin. Though they did not propose a mechanism for invasion, the authors did show that the invasion was related to the different type of M protein expressed by the different strains of S. pyogenes (LaPenta et al.). More recently, Thulin et al. demonstrated that streptococci can also invade and remain viable in phagocytotic cells, such as neutrophils and macrophages, in vivo. Again, these authors demonstrated that this invasion protects the bacteria from exposure to antibiotics, suggesting the evolutionary pressure behind the adaptation. Though the mechanism behind invasion is still not known, several more proteins have been identified as necessary for invasion, including streptolysin O, a secreted virulence factor, and SpeB, a protease that seems to cleave both human and bacterial proteins (Thulin et al. 1996). A similar situation is created by the secretion of Streptococcus pyogenes NAD-glycohydrolase (SPN), a protein that causes cell death once it enters the cell by disrupting metabolic pathways. Until recently, it was unknown how this enzyme was prevented from disrupting the same processes inside the bacteria, though Meehl et al. (2005) recently discovered a protein they named immunity factor for SPN (IFS), a SPN inhibitor found in the cytoplasm of GAS.

Figure 2. Schematic representation of the surface of S. pyogenes and some extracellular proteins. Adapted from Todar's Online Textbook of Bacteriology by Kenneth Todar. http://textbookofbacteriology.net/streptococcus.html. Copyright 2002. Used with permission.
Return to S. pyogenes home page
Davidson College Biology Home Page
Send any comments or concerns to beenglish@davidson.edu
© Copyright 2007 Department of Biology, Davidson College, Davidson, NC 28035