*This web page was produced as an assignment for an undergraduate course at Davidson College.*
Treatment & Future Research
HBV Prevention
Prevention is the best method to deal with HBV since there is no cure for the disease. The first became available in the U.S. in 1982 (Moyer and Mast, 1994). Currently, two monovalent hepatitis B vaccines are licensed in the U.S (CDC, 2006). The existing vaccines are made with recombinant DNA technology with HBsAg inserted into baker’s yeast. The vaccine must be administered in a 3 dose series of intramuscular injections. Studies have demonstrated that today’s vaccines are 80-95% effective in preventing HBV infection (CDC, 2006). For individuals who do become infected, even though there is no cure, there are treatment options. The figure below shows an individual being immunized with the HBV vaccine.
Figure 14. Image from http://newsimg.bbc.co.uk/media/images/41128000/jpg/_41128061_jab203.jpg
Treatment Options
The primary goal of HBV treatment is to permanently suppress viral replication in order to reduce hepatic necroinflammation and progression to liver fibrosis. A secondary goal is to obtain seroconversion to anti-HBe in HBeAg-positive patients. Currently, four drugs have been approved by the US Food and Drug Administration (FDA) for the treatment of chronic HBV. These drugs fall into two categories: those that directly interrupt viral replication and those that alter the immune response to the virus (Buster and Janssen, 2006).
Immunomodulatory Drugs
The first drug licensed for the treatment of chronic HBV was interferon (IFN) a-2b, which has immunomodulatory, antiproliferative, and antiviral properties. It was approved by the FDA in 1992. Because the drug must be administered subcutaneously, it is prescribed less frequently than oral medications. One advantage of IFN a-2b is that the course of treatment is finite, unlike many of the oral treatment options (Ocama et. al., 2005). Additionally, it is relatively effective, as it results in the loss of HbeAg in 25 to 40% of patients. In HBeAg-negative chronic HBV patients, this therapy is less effective, however, as IFN a-2b treatment results in a sustained immune response in only 24% of these patients. Current research focuses on the addition of a polyethylene glycol (PEG) molecule to IFN, which has been proven to significantly increase the half-life and prolong IFN activity. PEG-IFN a drugs have demonstrated increased levels of seroconversion in HBeAg-positive patients and sustained loss of HBe-Ag in 29 to 32% in HBeAg-positive patients. Improved viral responses were also observed in 36% HbeAg-negative HBV patients (Buster and Janssen, 2006).
Nucleos(t)ide Analogues
Nucleos(t)ide analogues are another treatment option for HBV. These drugs impair viral replication by directly inhibiting reverse transcriptase. Although these drugs can be administered orally, continued treatment must be provided to maintain viral suppression since this type of therapy seldom eliminates the virus (Ocama et. al., 2005). The first approved nucleoside analogue was lamivudine. Lamivudine is a cytosine analogue that inhibits reverse transcriptase by integrating into DNA chains and causing chain termination (Buster and Janssen, 2006). Although lamivudine has an excellent safety profile and is known to cause increased HBeAg seroconversion in HBeAg-positive patients, resistance to the drug occurs in approximately 24% of patients after treatment over 1 year and in 67% after treatment for 2 years (Ocama et. al., 2005). The figure below shows the chemical structure of lamivudine.
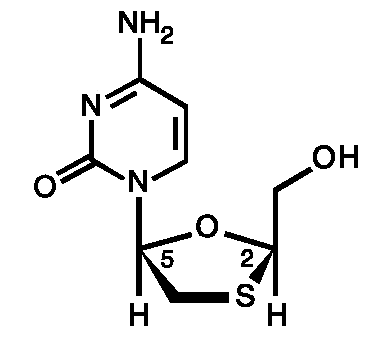
Figure 15. Image from http://www.stanford.edu/group/virus/hepadna/2005/lamivudine.gif
Adefovir dipivoxil, which is often prescribed once a chronic HBV patient has developed resistance to lamivudine, is the oral prodrug of adefovir, a nucleotide analogue of adenosine monophosphate. Through a mechanism analogous to that of lamivudine, adefovir terminates viral replication. Adefovir has been proven to be equally effective against both wild-type and lamivudine-resistant viruses, making it a good option for patients who have developed resistance to lamivudine. Although an effective immune response occurs while on the medication, response does not generally last after discontinuation of treatment. Though resistance to adefovir does occur, the incidence is less frequent in HBeAg-positive patients than for lamivudine (Buster and Janssen, 2006). Another treatment option if resistance to lamivudine occurs is entecavir, which is a guanosine analogue and a potent inhibitor of HBV polymerase. Entecavir only recently received FDA approval in the United States and has proven to be more effective than lamivudine in reducing serum HBV DNA (Ocama et. al., 2005). Research continues on several newer analogues to evaluate their effectiveness. Examples include telbivudine and emtricitabine, as well as drug combinations composed of drugs that act upon different stages of replication (Buster and Janssen, 2006).
Future Research
While researchers continue to search for the best first-line defense therapy for HBV infection, others must explore the notion of increasing access to HBV vaccination. Although HBV infection in the United States has decreased by 76% from 1987 to 1998, the virus continues to spread in populations where the vaccine is not available. As availability is increased, prevalence of the disease should decrease and lead to the eventual eradication of the virus in future generations (Ocama et. al., 2005).
Buster, E.H.C.J. and Janssen, H.L.A. 2006. “Antiviral treatment for chronic hepatitis B virus infection – immune modulation or viral suppression?” The Journal of Medicine 64 (6): 175-185.
Center for Disease Control. 2006. “Hepatitis, viral, type B.” Yellow Book – CDC Travelers’ Health. Accessed on 4 Feb 2007. http://www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=hbv.htm
Mast, L.A. and Moyer, E.E. 1994. “Hepatitis B: virology, epidemiology, disease, and prevention, and an overview of viral hepatitis.” American Journal of Preventative Medicine 10: 45-55.
Ocama, P., Opio, C.K., and Lee, W.M. 2005. “Hepatitis B virus infection: current status.” The American Journal of Medicine 118 (12): 1413-1422.
Return to Christie's Immunology Home Page
Christie Brough. Biology 307: Immunology. Dr. S. Sarafova. Davidson College. May 4, 2007.