**This webpage was produced as an assignment for an undergraduate course at Davidson College**
Life Cycle of Herpes Simplex Virus
Structure
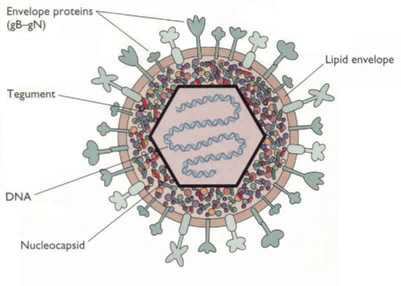
Herpes Simplex Virus Type (HSV) is a double stranded DNA virus that belongs to Herpesviridae family (Smiley et al. 2004). It contains three main structural components. A central core holds the viral DNA, an inner core is surrounded by an envelope that is made of viral glycoproteins and host cell membranes, and a capsid. The tegument is located between the capsid and the envelope (Spear et al. 2004) and various proteins that are delivered into the infected cell upon cell fusion (Smiley et al. 2004). (see Figure 1).

Figure 1. HSV structure. Image from http://www.bact.wisc.edu/themicrobialworld/hsv1struc.jpg
Genes
The HSV genome encodes for over 80 proteins (Khanna et al. 2004). There are three classes of viral genes that are transcribed and translated in a specific order, including the immediate early (IE; α), early (E; β), and late (L; γ) genes (Jenkins and Turner 1996). Immediate early genes (such as α-TIF) are transcribed and translated first. Four (ICP0, ICP4, ICP22, and ICP27) of these five proteins from the IE genes serve as regulatory proteins that initiate transcription of early genes by the host cell’s RNA polymerase (Smiley et al. 2004). ICP4, however, is the main regulatory protein of HSV (Jenkins and Turner 1996). Early genes serve to downregulate immediate early gene expression and upregulate the third set of genes in infection, called late genes (see Figure 2). Late genes downregulate early gene expression and are structural proteins (Smiley et al. 2004). After the late phase of infection, a viral capsid is formed in the nucleus that contains viral DNA. The capsid buds through the nuclear membrane and leaves the cell through the Golgi complex. During this process, the virus acquires its tegument and envelop, and the host cell dies as virus is released. HSV is then retrogradely transported along axons to the cell body of neurons to establish a latent infection. This transport is accomplished by dynein and dynactin, which move HSV capsids along microtubules (Dohner et al. 2002).
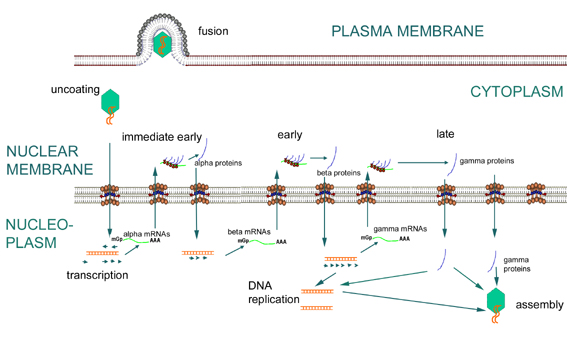
Figure 2. HSV replication. Image from http://pathmicro.med.sc.edu/mhunt/dna1.htm
Primary Infection: The Lytic Cycle
HSV infects its host through both lytic and latent infection, and replication of HSV occurs within 15 hours after infection (Jenkins and Turner 1996). In the lytic cycle, HSV infects epithelial cells located in the mucosa, replicates, and causes epithelial cell death (Smiley et al. 2004; see Figure 3). HSV-1 most frequently invades oral and ocular epithelial cells while HSV-2 infects the genital areas, but both strains have the ability to cause infection in either area of the body (Herbst-Kralovetz et al. 2006). In order to infect epithelial cells, glycoproteins (namely gB, gC, and gD) on the surface of HSV fuse with entry receptors on the host cell membrane. One study demonstrated that when the virion lacks gC, the virus loses some function in binding to host cells, and the infectivity of the virus is decreased by a factor of 10. Also, gC-negative virions did bound to host cells required much more time to penetrate the host cell compared to controls (Herold et al. 1991). gD has also been found to be necessary for fusion between the host cell membrane and the virus envelop and for entry of viral particles into the host cell (Spear et al. 2004).
There are three known types of entry receptors located on the host cell that bind to HSV during cell infection and fusion. Heparan sulfate is a glycosaminoglycan (GAG), and it binds to gB and gC (Spear et al. 2004). Another entry receptor is herpesvirus entry mediator (HVEM), which is a member of TNF receptor family. This receptor is expressed at high levels on NK-T cells and naïve CD8+ cells and at weaker levels on CD4+ cells and dendritic cells. HVEM is also expressed on B cells, epithelial cells, and fibroblasts, so HSV has the potential to infect any of these cell types. The third receptors are nectin-1 and nectin-2, which are members of the immunoglobulin superfamily, and these receptors are expressed in epithelial cells, fibroblasts, and neurons. HSV-1 and 2 both bind to HVEM and nectin-1, while HSV-1 also uses heparan sulfate and HSV-2 uses nectin-2 (Spear et al. 2004).
Latency
In order to establish latency, the virus enters sensory neurons, which are non-dividing, and replicates infrequently (Smiley et al. 2004). Latency-associated transcripts (LAT) are the only mRNAs that are expressed during latency, and these proteins serve to help the neuron survive initial infection. LAT are also required to establish and maintain viral latency and for the virus to be reactivated from latency (Bystricka and Russ 2005).
Although the adaptive immune response is present, the virus is occasionally reactivated and when this occurs, it anterogradely travels back to epithelial cells to form herpetic lesions (Spear et al. 2004). Reactivation can be caused by numerous factors, including stress, ultraviolet light, heat, fever, hormonal changes, menstruation, and physical trauma to the neuron (Jenkins and Turner 1996).
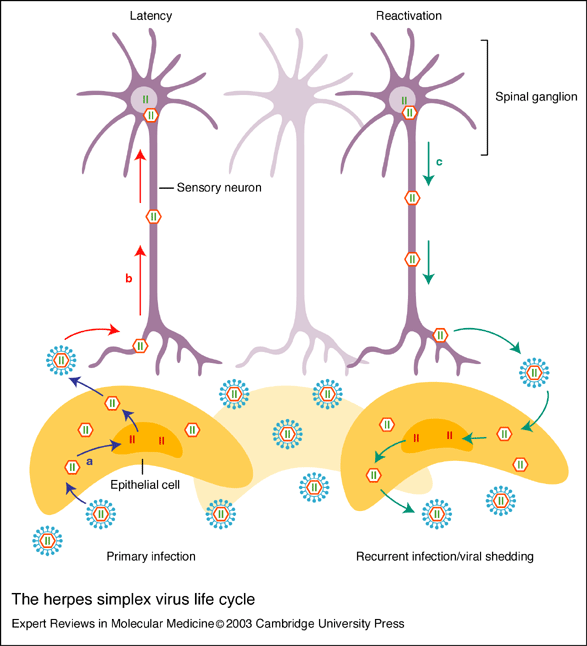
Figure 3. HSV lifecycle. Image from http://www-ermm.cbcu.cam.ac.uk/03006987h.htm
Davidson College Biology Department
Contact jehodge@davidson.edu with questions or comments.