The Life Cycle of Neisseria meningitidis
Neisseria meningitidis is a gram-negative, aerobic, nonmotile, coccus bacterium, known most commonly and notoriously as a leading cause of bacterial meningitis in humans. Structurally, the bacertium ranges in size from 0.6 to 1.0 µm and is characterized by its antiphagocytic polysaccharide capsule. Neisseria meningitidis bacteria are divided into thirteen seperate serogroups based upon variation in the capsule, with serogroups A, B, C, Y, and W135 responsible for the majority (>90%) of meningococcal meningitis and meningococcemia cases (Yazdankhak et al., 2004). Interestingly, researchers have noted that approximately half of all strains that are isolated from carriers lack the polysaccharide capsule (Claus et al., 2002); for years it had been thought that this caused the bacterium to be non-pathogenic, however more recent research has shown that N. meningitidis has the ability to regulate its capsule production - something which will be discussed in greater depth later (Swartley et al., 1997).
Infection by N. meningitidis will typically result in one of two disease variations, meningococcemia or meningococcal meningitis; the latter is notorious for its very high mortality rate (Todar's, 2007).
The bacteria's primary means of human infection occurs via inhalation, allowing for attachment to the epithelial cells found in the oropharyngeal and nasopharyngeal mucosa; after this point the bacteria then crosses the mucosal barrier and enters the body's bloodstream (Todar's, 2007). Being an exclusively human-infecting bacterium, N. meningitidis's most natural habitat is the nasopharynx, and its infections can occur as either endemic or epidemic (Taha, 2006). Several studies have shown that approximately 10% of the general population are asymptomatic carriers; these individuals are thought to be the primary source of the pathogenic strains (Yazdankhak et al., 2004). Additionally, the rates for both carriage and trnasmission a greatly increased within small or closed populations, such as college and university students (Olcén et al., 1981). While the carrier stage varies considerably in length from one individual to the next, the N. meningitidis will eventually colonize the oropharynx which in turn produces the antibody response from the three major Ig classes within the first few weeks (Kremastinou et al., 1999); it is from this point where the bacterium will cross the aforementioned mucosal barrier, allowing for a significant number of various diseases to develop (Yazdankhak et al., 2004). By far the most prevalent disease is that of meningococcal meningitis, although septic arthritis, pneumonia, conjunctivitis, purulent pericarditis, otitis, sinusitis and urethritis are all rare but highly possible (Yazdankhak et al., 2004).
So what specifically makes N. meningitidis toxic? The lipopolysaccharides (LPS) happen to be a major component of the bacterium's virulence; it acts by inducing pro-inflammatory mediator production, which ultimately leads to septic shock (Yazdankhak et al., 2004). A unique aspect of the LPS of N. meningitidis is that they lack the polysaccharide O-antigens, which are normally found repeating in enteric bacteria; resultantly, the LPS in this case are commonly referred to as lipooligosaccharides (LOS) (Yazdankhak et al., 2004). This endotoxic mechanism is effective in that N. meningitidis undergoes autolysis during growth periods and releases portions of its cell membrane into its surrounding environment in a soluble, toxic form (Todar's, 2007). Purified LOS is very toxic and surpresses leukotriene B4 synthesis in luekocytes, as well as negatively affecting numerous other biochemical interactions and pathways (Todar's, 2007).
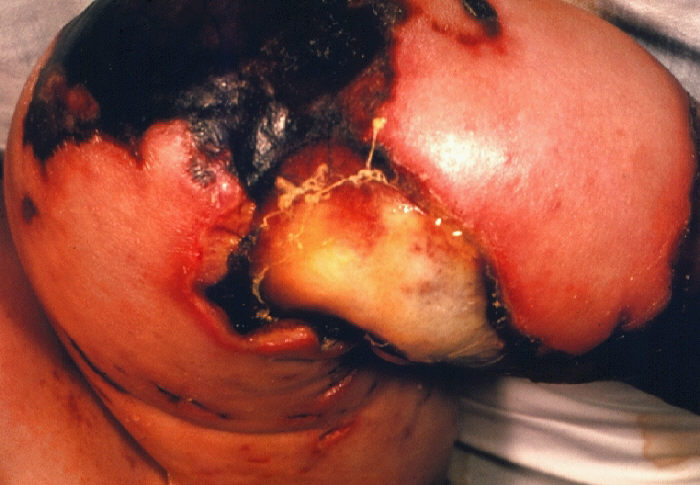
The following is a photograph of gangrene that has resulted from infection by N. meningitidis, this instance in the form of meningococcemia:

"4 month old female with gangrene of knee due to meningococcemia." Courtesy of CDC/Mr. Gust.
[http://phil.cdc.gov/Phil/home.asp] Accessed 02/11/07.
References:
Claus, H., et al. "Many carried meningococci lack the genes required for capsule synthesis and transport." Journal of Microbiology 148 (2002): 1813–19.
Kremastinou, J., et al. "Detection of IgG and IgM to meningococcal outer membrane proteins in relation to carriage of Neisseria meningitidis or Neisseria lactamica." FEMS Immunology & Medical Microbiology 24 (1999): 73–78.
Olcén, P., Kjellander, J., Danielsson, D. & Lindquist, B. L. "Epidemiology of Neisseria meningitidis; prevalence and symptoms from the upper respiratory tract in family members to patients with meningococcal disease." Scandinavian Journal of Infectious Diseases 13 (1981): 105–109.
Swartley, J. S., et al. "Capsule switching of Neisseria meningitidis." Proceedings of the National Academy of Sciences 94 (1997): 271–76.
Taha, Muhamed-Kheir. "Worldwide tracking of Neisseria meningitidis." Journal of Postgraduate Medicine 52.1 (2006): 29.
Todar, Kenneth. "The Pathogenic Neisseriae." Todar's Online Textbook of Bacteriology, University of Wisonsin - Madison, Department of Bacteriology. <http://textbookofbacteriology.net/neisseria.html> Accessed 02/11/07.
Yazdankhak, Siamak P., and Dominique A. Caugant. "Neisseria meningitidis: an overview of the carrier stage." Journal of Medical Microbiology 53 (2004): 821-32.
Links:
Email questions or comments to Jordan Sullivan.