Neurodegenerative disorders, such as AlzheimerÝs and ParkinsonÝs disease, degrade the central nervous system resulting in senile dementia and motor dysfunction. Currently there are preventative and therapeutic measures for AlzheimerÝs disease but no cure. Acetylcholine-esterase inhibitors and antioxidants are currently employed to slow or prevent the disease process. These treatments, however, do not solve the underlying degeneration of the nervous system. Novel techniques involving in situ gene expression, however, show the capability of regenerating nerve cells (Neotherapeutics "AIT-082", 1997).
Prior research has established that neurotrophic factors regulate nerve cell growth (Neotherapeutics "AIT-082", 1997). Direct administration of these factors to animal cell cultures prevents and/or reverses nerve degeneration. Unfortunately, because of their large size neurotrophic factors cannot cross the blood-brain barrier. Consequently,direct injection, a method requiring drilling into the patientÝs head, must be employed. Novel purine analogs, however, have shown the capability of increasing neurotrophin production, while being small enough to cross the blood-brain barrier (Glasky, 1992).
AIT-082
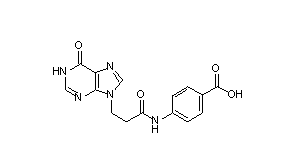
One such purine analog is AIT-082, 4-[[3-(1,6-dihydro-6-oxo-9H-purin-9-yl)-1-oxopropyl]amino]benzoic acid. AIT-082 is the first compound that has entered clinical trials that has demonstrated activation of multiple genes to produce three different neurotrophic factors (Nerve Growth Factor (NGF), Neurotrophin-3 (NT-3), and basic Fibroblast Growth Factor (bFGF)) in the specific areas of the brain associated with memory loss. Furthermore, AIT-082 has the advantage of being rapidly absorbed and active after oral administration (Neotherapeutics "First", 1997).
AIT-082 augments mammalian cellular and neural activity through the modulation of carbon monoxide-dependent guanylyl cyclase by selectively and controllably inducing genetic expression in vivo (Glasky, 1996). Research indicates that nitric oxide (NO) and carbon monoxide (CO) may function as neurotransmitters. CO is produced bythe enzyme heme oxygenase II (HO). Research suggests that when CO diffuses into a neuron, it induces a rise in cyclic guanosine monophosphate (cGMP), by modulating the enzyme guanylyl cyclase (Glasky, 1996). The rise in cGMP modifies other neurotrophic factors and translates into the production of NGF, NT-3, and bFGF. These factors in turn regenerate neurons and aid in their survival (Glasky, 1996).
AIT-082 (Fig. 1) is comprised of two molecules, hypoxanthine and p-aminobenzoic acid (PABA), with different chemical properties. Hypoxanthine (Fig. 2) displays a slight neurological effect (Manzke and Gustmann, 1989), and an immunological effect (Glasky, 1992). When, however, hypoxanthine is coupled with PABA it shows a different, more pronounced neurological effect and a decreased immunological effect. Hypoxanthine is also actively transported across the blood-brain barrier (BBB) by a saturable transport system (Spector, 1987). The BBB is a tight junction of cells that prevents intercellular diffusion. To move across the BBB, receptor mediated and pore mediated vesicular transport are used but most frequently, movement is via transcellular passive transport (Elo et al., 1982). The ability to be moved across the BBB may be an important role of hypoxanthine in the efficacy of AIT-082.

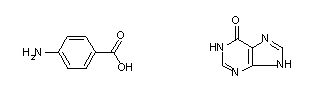
Not to be overlooked is the three-carbon bridge that keeps hypoxanthine and PABA together so that they may work in concert. AIT-082 shows a synergistic in vivo effect different than the individual effects of hypoxanthine and PABA (Glasky, 1992). This suggests that the bridge maintains the linkage of the two groups during interaction at itssite of action and is critical for function.
Purifying the AIT-082 receptor
In addition to its potential therapeutic benefits, AIT-082 can also serve as a molecular probe used to elucidate the pathology and genetics involved in neuronal degeneration and regeneration. AIT-082 acts as a genetic trigger by interacting with a protein(s) that in turn initiates a cascade of molecular events culminating in the in situ production of neurotrophic factors.
The pursuit of drug receptors has been carried out for years. In 1973, Pert and Snyder identified and located an opiate receptor in mammalian nervous tissue. They used tritiated naloxone, a powerful opiate antagonist, to isolate the receptor using mice and rat brain homogenates and guinea pig intestinal homogenates. By using other competitive and noncompetitive opiate antagonists to displace the tritiated probe in conjunction with subcellular fractionation and brain partitioning, Pert and Snyder were able to purify the mammalian opiate receptor. Their use of a drug as a probe to find an endogenous protein receptor is fundamental to my experimental approach. Though I have not used AIT-082, I am using PABA as a ligand to isolate endogenous protein(s).
Though PABA is only part of AIT-082, it independently can serve as an
effective ligand to isolate the receptor of AIT-082. The concept of multiple
binding site proteins supports the idea that PABA alone can carry out this
task. The interaction between a protein and its substrate relies on the
unique three-dimensional conformations of the protein and substrate. Since
these interfaces are made possible by ionic interactions such as weak hydrogen
bonding, many close bonding surfaces are required to facilitate molecular
recognition between a protein and a substrate. Proteins accommodate particular
substances by forming pockets in their surfaces made by their secondary,
tertiary, and quaternary structure. These pockets or sites create specific
environments that house some substances and not others. Often a protein
will have more than one binding site that may be selective for a substrate
different from a neighboring site on that same protein. The scenario may
be thought of as a door with multiple locks that requires specific keys
for each lock before the door will open. The linking of two compounds,
each of which independently interacts with a particular protein, increases
the aggregate number of hydrogen bonds between the compounds and the protein,
allowing for bio-activation. By getting all the keys in the respective
locks at the same time and unlocking
them simultaneously, the door will open.
Under this premise either PABA (Fig. 2) or hypoxanthine (Fig. 2) alone could serve as a ligand to identify the protein receptor of AIT-082. Certainly, using AIT-082 as the ligand would be the better method but the drug is not commercially available and attempts at de novo AIT-082 synthesis proved inconclusive. I have, therefore, used a modular approach employing PABA as a probe to determine AIT-082Ýs protein receptor.
I used affinity chromatography to isolate a protein from a general mammalian brain homogenate. The fundamental principal composing the affinity method is the isolation of a particular protein by chromatography on a bio-ligand that has been immobilized on an inert matrix. The method relies on the specificity of a protein binding site for a particular ligand. Affinity chromatography allows protein purification in a relatively short time with a high yield. It simplifies the isolation process by using the preexisting ligand binding relationships.
It is the present scope of this study to isolate the protein receptor of PABA from mammalian nervous tissue by means of affinity chromatography. The greater aim of the study is to eventually determine the definitive protein receptor of AIT-082 for application toward the study of neurodegenerative diseases.