Compare with figures 7.24 and 7.25 in the Campbell text.
Focused Reading: "Microtubules..." pp 88-89
stop @ "Centrioles"
figures 4.21 on page 86
Goals for this Lab:
During this lab, you will collect data on the regeneration of
flagella on Chlamy. These cells will have been deflagellated before
you come to lab and you will measure the length of the flagella
over a one hour time period. You will learn how to use a version
of NIH Image and the computer to capture images of the cells and
measure their flagella on the computer moniter.
This set of experiments requires a lot of teamwork. You should have already decided who will do which job(s) in order to make the necessary observations, and record all the information. [However, in the interest of your own edification and getting more bang for the buck, each person should sneak time to make observations for yourself since there will be test questions which are based on your laboratory work.] Do not waste time at the beginning of lab. The cells have just lost their flagella which means they cannot follow the best light in order to eat, or escape predators. They will start regenerating their flagella ASAP. On the other hand, do not begin the experiments below until you are ready - you may have to repeat the entire process if you begin before organizing yourselves because, "I thought you were keeping the time!"
You will want to measure the length of the flagella as a function of time. At each time point, two people should each measure the length of 20 pf14 flagella (total of 20 flagella on 20 cells). Once the cells have been fixed with Lugol's solution, the data are safe and you do not need to rush. With this in mind, you might want to rotate the job of measuring flagella. You should organize yourselves so that aliquots are fixed every 5 minutes. You will use the same ingredients, reagents, as last week so stain them with Lugol's to fix the cells and flagella and to enhance visualization.
A) I have laboriously removed the flagella from about 5.0 X
1012 pf14 cells
How many flagella did I pile up? (For those with time on their
hands,
how close to the moon would this stack go if they were placed
end to end?)
B) Since there are 4 people in a group, each person should
have a job:
#1 - One person should record all the data and and make sure that
no time points are missed.
#2 - One person should be the time keeper and fix all the aliquots
at the right time.
#1 and #2 should prepare all the slides for #3 and #4. Do not
make the slides until they are ready for them. If you leave Chlamy
cells under a cover slip too long, they will pop off their flagella
again.
#3 and #4 - Two people should measure the length of the fixed
pf14 flagella.
C) Person #1: set up 5 microfuge tubes and label them
0 - 60 at intervals of 15. In each tube there should be 50 µl
of Lugol's fixative. Have a table ready that has spaces for all
the data he or she will need to record.
Person #2: As soon as your group is ready, add 50 µl
of deflagellated cells to the appropriate microfuge tube and move
the flask back to the light shelf. Call this time zero. This is
the starting length of the pf14 flagella.
People #3 and #4: measure the flagella lengths from the
fixed pf14 ; 20 flagella from 20 cells.
D) This cycle of events should happen every 15 minutes, so there will be a total of 5 time points and data for each. Do NOT record averaged data in your lab book. Enter the raw data; there will be time to average the results later and a good scientist keeps all data, not just the averaged results. If you were doing AIDS research and you recorded only averaged results, you might find yourself in jail.
E) After one hour has passed, you should have collected all the data. The last step in any experiment is to clean up. You should clean your area, turn off the microscopes, throw away any trash, and return any equipment to where you found it. This is important since so many people use this equipment and room. If you get an opportunity to work in a research lab, you had better clean up and return things properly or you may find yourself unemployed.
| Time | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 0 | ||||||||||||||||||||
| 15 | ||||||||||||||||||||
| 30 | ||||||||||||||||||||
| 45 | ||||||||||||||||||||
| 60 |
To surgically remove the flagella of Chlamy, you can either have great eye-hand coordination, or use the pH shock method. As it so happens, Chlamy is very sensitive to changes in its environment. If we manipulate the pH of the growth medium by adding acetic acid until the pH falls from about 7.2 to 4.5, the cells shed their flagella like nobody's business. Scientists have made it their business to figure out why this happens. We know that cells will not shed their flagella if there is no calcium in the growth medium. (Calcium can be removed from any solution by adding in a compound commonly referred to as EGTA. EGTA has a very high affinity for calcium and acts as a chelator, like a molecular sponge, to ionically absorb all the calcium which means Chlamy cells can't use or sense any calcium ions if EGTA is present.) Another experiment has shown that if you can experimentally elevate the level of calcium in the cytoplasm of Chlamy cells, they shed their flagella. Hypothesize what is going on when Chlamy cells shed their flagella when pH shocked in the presence of calcium. Can you devise an experiment to test your hypothesis?
By now you may be wondering why anyone would care how long it takes pond scum to regrow its flagella. Chlamy is a model organism for studying flagella and much of our understanding of cilia and flagella is due in large part to our understanding of Chlamy flagella.
Well, there is an interesting story of colossal proportions
that you must figure out. As you read in Campbell, the flagella
are comprised of many different proteins (about 200 different
proteins are required to make a normal flagellum) but the predominant
one is tubulin. Each Chlamy flagellum is built upon the
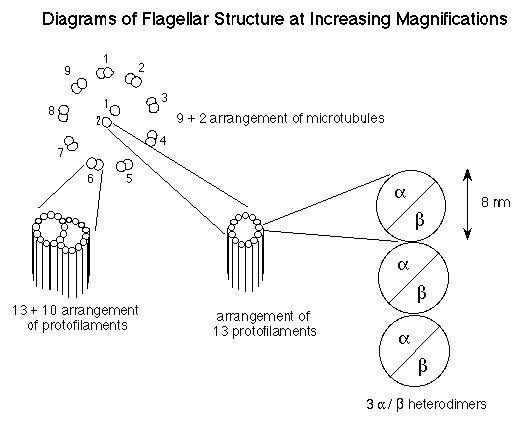
9+2 structure of microtubules (see the figure below). The outer
9 microtubels are "doublets", consisting of a complete
circle of 13 protofilaments fused with a partial circle of 10
protofilaments. The 2 centrally located microtubules consist of
a "singlet" of 13 protofilaments. Therefore, each flagellum
contains (9x(13+10)) + (2x13) protofilaments. Each protofilament
is composed of dimers of a tubulin and b tubulin.
Each monomer of a globular tubulin molecule has a 4 nm (4.10-9
meters) diameter and is comprised of 450 amino acids. Therefore,
the a /b dimer has a diameter of 8 nm and is made
of 900 amino acids. Although there are multiple genes for tubulin,
for the sake of simplicity let's assume a single gene for each
form of tubulin (one for a and one for b). These prototypical
genes are about 1800 bases long.

Here are some questions for you to answer:
1) Based on your results for flagella regeneration, calculate how many amino acids are being polymerized per minute into the tubulin component of the regenerating flagella.
2) Assume that all of the mRNA needed for this process is being synthesized de novo, from scratch. How many mRNA bases (assume no introns) must be transcribed per minute if every mRNA is translated only once? What if each mRNA is translated 100 times?
3) If RNA polymerase can travel no faster than 2500 bases per minute, is it possible for all of the RNA to be transcribed de novo?Explain your answer.
You will not be able to answer these questions with your experimentally determined rate since those will not be determined for another two weeks. However, make the assumption that they grow at 0.17 µm per minute. Once you have determined the rate in your experiment, you can try the calculations again.
This is where each lab group has to formulate an hypothesis and design an experiment to test that hypothesis. To formulate an hypothesis, you might just wonder aloud, "What if we....?" For instance, what if we prevent the cells from transcribing any new RNA? What if we prevent the cells from translating any new proteins? What if these plant cells are put in the dark? Would gametes (in G0) regenerate flagella faster or slower than vegetative (mitotically active) cells? What would happen in the presence of added ATP? caffeine? glucose? amino acids? EGTA? Once you have found a question that interests you, you should then use your knowledge of molecular and cellular biology to formulate an answer to your question. For example, if we block translation with cycloheximide, then you might hypothesize that flagella will not grow at all. This is a good hypothesis; a good hypothesis can be tested. A bad hypothesis might be, "Chlamy cells do not like to have their flagella removed and are happier when the flagella are regenerated." How could this be tested?! Formulate your hypothesis so that you can design an experiment to test it.
| NAME | FUNCTION | STOCK | FINAL |
| cycloheximide | translation inhibitor | 2 mg/ml | 10 µg/ml |
| actinomycin D | transcription inhibitor | 5 mg/ml | 50 µg/ml |
| caffeine | cyclic nucleotide (cAMP) phosphodiesterase inhibitor |
66 mM | 6.6 mM |
| arginine | essential amino acid | 10 mg/ml | 100 µg/ml |
| calcium | signal transduction/ second messenger | 100 mM | 1 mM |
| lithium chloride | disrupts production of | 1 M | 20 mM |
Remember to include good controls in your design. A good control
is an experimental condition that will give you a standard or
predictable result against which you can compare the results of
the condition you are actually interested in. For example, if
you wanted to see the effects of disco music on the regeneration
of flagella, you would design an experiment that had 2 experimental
conditions:
1) cells regenerating their flagella in the presence of disco
music
2) control cells regenerating their flagella in the presence of
pleasant, non-disco music
(Notice the difference between the control and experimental is
only one variable - the presence or absence of music.)
Your hypothesis would probably be that disco will prevent flagella from growing. This is a testable hypothesis because you can measure the length of the flagella in the two situations (plus and minus disco). Let's look at a hypothetical set of results. When the cells are subjected to disco, the flagella did not grow. When the cells were grown in the presence of normal music, they did not grow either. How should these results be interpreted? Did disco prevent the regeneration? What do the results of your control condition tell you? Why must every experiment have good controls?
Each group should decide upon a question to answer next week, formulate an hypothesis, design the experiment, and discuss the protocol with me. This gives us a chance to answer any major questions you might have and order the reagents you will need.
Your written protocols must be turned in today and they should be specific enough so that next week, you can come into the lab and begin immediately by following your own directions. We will look over your protocols and give them back to you at the next lab meeting.
You should also have the following in your lab notebook:
1) The table of data which has all the data from today's experiment.
2) Answer all the questions asked of you in the protocol above
(with the exception of the optional question regarding the moon).
3) You should note any observations you think note worthy - use
your best judgment.
© Copyright 2000 Department of Biology,
Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu