Week of Semester |
Subject Matter and
Assignments Due |
| Week 1: Aug 25 & 27 |
Discuss: semester-long research plans & set educational goals Discuss: domains of life, genome sequencing, JGI and our species Halomicrobium mukohataei genome sequence home page Halomicrobium mukohataei genome portal Standard Operating Procedure (SOP), Quality Control (QC), and Triage How many do we want to compare? Eight? Sixty-four? |
Read the paper from last year's course here: Bakke et al., 2009. Read the three papers published on our species. Start with RNA genes first and ID species specific Shine-Dalgarno sequence Amino Acids Table (learn 1 letter code) |
|
Week 2: |
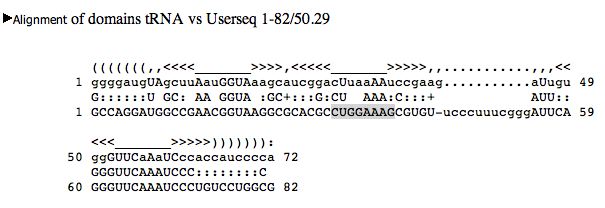
Examine RNA gene results: Sanger sequence checks
Identify Shine-Dalgarno sequence for our
genome. Look in large ribosome subunit genes (LSU), DNA polymerase
subunits, RNA polymerase subunits. Collect consensus results. If Shine-Dalgarno is missing for a particular gene, is the gene part of an operon? Find genes that do not have a perfect Shine-Dalgarno sequence. What can we do about these exceptions? 1) Verify start codon: 2) Verify Shine-Dalgarno sequences: See examples from a different species (Ammonifex) |
Read: Armbrust et al. 2004. The Genome of the Diatom Thalassiosira pseudonana: Ecology, Evolution, and Metabolism. Science 306: 79 - 86. RNA and Shine-Dalgarno Presentations + 1 option by Dr. C. Controlled vocabulary Problems to be addressed: Pseudogenes, transposons, horizontal gene transfer, orthologs, paralogs, homology, hypothetical genes, unknown function, quality of data for annotation. Establish SOP (standard operating procedures) for genes. Databases and Tools: BLAST, CDD, KEGG, BioCyc, Tcoffee, EC numbers, and phylogenetic trees |
|
| Week 3: Sep 8 &10 |
Compare genome by 3 annotation services The SEED for automated annotation and viewing Manatee for automated annotation (JCVI) Work on annotation projects - smaller scale |
| Work in groups to choose first research project - smaller scale | |
| Week 4: Sep 15 & 17 |
Finalize plan and begin project- smaller scale Make clear the goals for each person |
Read halophile genomics paper by Baliga et al., 2004 Discuss end goals and methods for accomplishing this |
|
| Week 5: Sep 22 & 24 |
Continue projects 10 glossary entries for each student (graded by Dr. C.) |
| Continue projects - smaller scale | |
| Week 6: Sep 29 & Oct 1 |
Continue projects - smaller scale |
Each person's portion of project due Oral
presentations with peer review |
|
| Week 7: Oct 6 &8 |
Conclude small scale
projects What types of whole-genome projects are feasible? |
First
methodology tutorial Due (graded by Dr. C.) |
|
Week 8: |
Fall Break |
Begin whole-genome projects with clear roles defined |
|
Week 9: |
Continue whole-genome projects |
|
SOP for whole-genome projects Continue whole-genome projects |
|
| Week 10: Oct 27 &29 |
Tutorial #2 Assignments: due Nov. 12 Olivia - perl script to compare proteomes Download Format Converter and convert all proteomes |
| Continue whole-genome projects | |
| Week 11: Nov 3 & 5 |
Continue whole-genome projects Design experimental testing |
| Continue research | |
| Week 12: Nov 10 & 12 |
Continue research |
Pathway tutorial Due (graded by Dr. C.) |
|
| Week 13: Nov 17 &19 |
Continue research |
Assess Status and Agree on Endgame Write the final paper |
|
Week 14: |
Oral Presentations on
your whole genome story |
First draft of final paper (web
site) due |
|
| Finish final paper | |
| Week 15: Dec 8 |
Final
draft of final
paper (web site) due |
| No final exam |