Graves' disease is a thyroid-specific autoimmune disorder in which the body
makes antibodies to the thyroid-stimulating hormone receptor (TSHR), leading
to hyperthyroidism, or an abnormally strong release of hormones from the thyroid
gland. Normally, the release of thyroid hormones is mediated by thyroid-stimulating
hormone (TSH), a hormone secreted by the pituitary gland that binds to TSHR
to stimulate the thyroid to release thyroid hormones. This normal cycle is self-regulating:
the hormones secreted by the thyroid keep more TSH from being produced (Janeway,
2001).
The autoantibodies produced in Graves' disease are not subject to negative feedback,
so they continue to be produced and bind to TSHR even when thyroid hormone levels
rise too high. These antibodies act as agonists, stimulating more hormones to
be released and thus leading to hyperthyroidism.

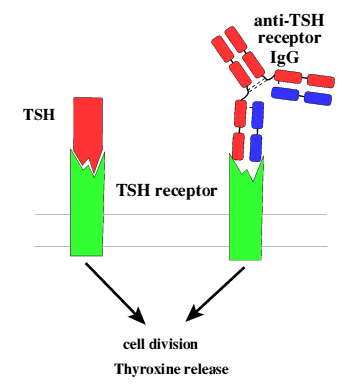
Figure 1: Both TSH and autoantibodies can stimulate the TSHR
on thymocytes
to induce release of hormones such as thyroxine. Figure taken with permission
from Holmes,
2001.
View a Chime Image of the proposed structure of the TSHR ectodomain. Image created by Kajava (1998).
Patients with Graves' disease can exhibit a variety of symptoms related to hyperthyroidism, including diffuse goiter (enlarged thyroid gland), rapid pulse, weight loss and trembling. In addition, some patients with Graves' disease exhibit symptoms unique to this form of hyperthyroidism, including ophthalmopathy (bulging eyes) and rarely, pretibial myxedema (swelling of shins) (ATA, 2003). The symptoms of Graves' disease stem partly from hyperthyroidism and partly as a consequence of autoimmunity.

Figure 2: Patient with a large goiter, prominent on the left
side of the neck.
Figure taken with permission from www.thyroidimaging.com
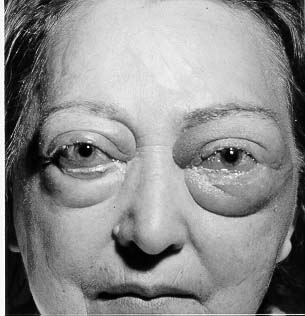
Figure 3: Patient with opthalmopathy.
Figure taken pending permission from www.thyroidmanager.org
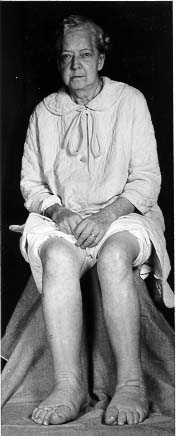
Figure 4: Patient with pretibial myxedema.
Figure taken pending permission from www.thyroidmanager.org
Several factors can influence a person's susceptibility to Graves' disease, although the mechanism of initiation remains unknown. Females are much more likely to contract the disease than males, with the highest-risk ages for either sex being between 40 and 60 years (Weetman, 2000). Thus, hormone levels are probably important in the initiation of the disease. White and Asian populations are at higher risk than black populations. Often, the onset of Graves' disease is preceded by a traumatic event, implicating that stress may play a role in initiation. Smoking is also a significant risk factor; it is most strongly associated with the development and persistence of ophthalmopathy in Graves' disease patients, even after treatment (Bartalena et al., 1998).
Genetic factors are important, but not determinative, for predisposition to Graves' disease. Twin studies show a 20 percent concordance rate between monozygotic twins with the disease and lower rates among dizygotic twins and HLA-identical siblings. Several HLA alleles have been shown to predispose certain groups of people to the disease, with regional and racial variation (Weetman, 2000). Among the alleles implicated among British Caucasians are HLA class II alleles DRB1-0304, DQB1-02, and DQA1-0501 (Heward et al., 1998). The association of HLA alleles with susceptibility to Graves' disease is typical of an autoimmune disorder. Allelic affinity for different peptides directly influences which self-peptides will be produced and how likely it is that they will be recognized by T cells.
Genetic analysis has shown that polymorphisms in the cytotoxic T-lymphocyte antigen 4 (CTLA-4) gene are associated with Graves' disease (Weetman, 2000). CTLA-4 is a receptor expressed on activated T cells that inhibits T cell proliferation by binding to the costimulatory B7 molecule and preventing further activation. The polymorphisms linked to Graves' disease may correspond with lessened effectiveness of this inhibitory receptor (Kouki et al., 2000). CTLA-4 has also been linked to other organ-specific autoimmune diseases, indicating that it may play a general role in autoimmunity.
Another gene that Pani, et al. (2002) have linked to Graves' disease is the vitamin D-binding protein (DBP) gene. Graves' disease patients have much less vitamin D in their bloodstream than healthy individuals. Pani, et al. found that in a study of several families, a polymorphism of the DBP gene was associated with susceptibility to Graves' disease but not Hashimoto's thyroiditis, a hypothyroid illness. The mechanism whereby DBP could affect autoimmunity is unknown, but such a relationship indicates that vitamin D regulation might be important in Graves' disease.
Many of the symptoms of Graves' disease are directly related to its cause: organ-specific autoimmunity. For example, the goiter that is typical of Graves' disease is caused by swelling of the thyroid due to inflammation and lymphocyte infiltration. Lymphocytes within the thyroid contribute to further autoimmune responses, and the thyroid cells themselves interact with the immune system to exacerbate the symptoms of hyperthyroidism. These thyrocytes express proteins they normally would not, including HLA class II molecules, CD40, adhesion molecules such as CD54, and cytokines such as TNF-a, IFN-g, IL-1 and IL-6. Germinal centers can form within the thyroid tissue of affected individuals, exacerbating the autoimmune response and causing further inflammation (Weetman, 2000). Likewise, the ophthalmopathy associated with Graves' disease is due to retroorbital swelling from lymphocyte and macrophage infiltration. Orbital fibroblasts are known to secrete TSHR, so presumably the recognition of TSHR antigen leads to this inflammatory response. However, the mechanism for this specific response to the extraocular muscles is puzzling, since other antigen-producing preadipocytes throughout the body remain untargeted (Weetman, 2000).
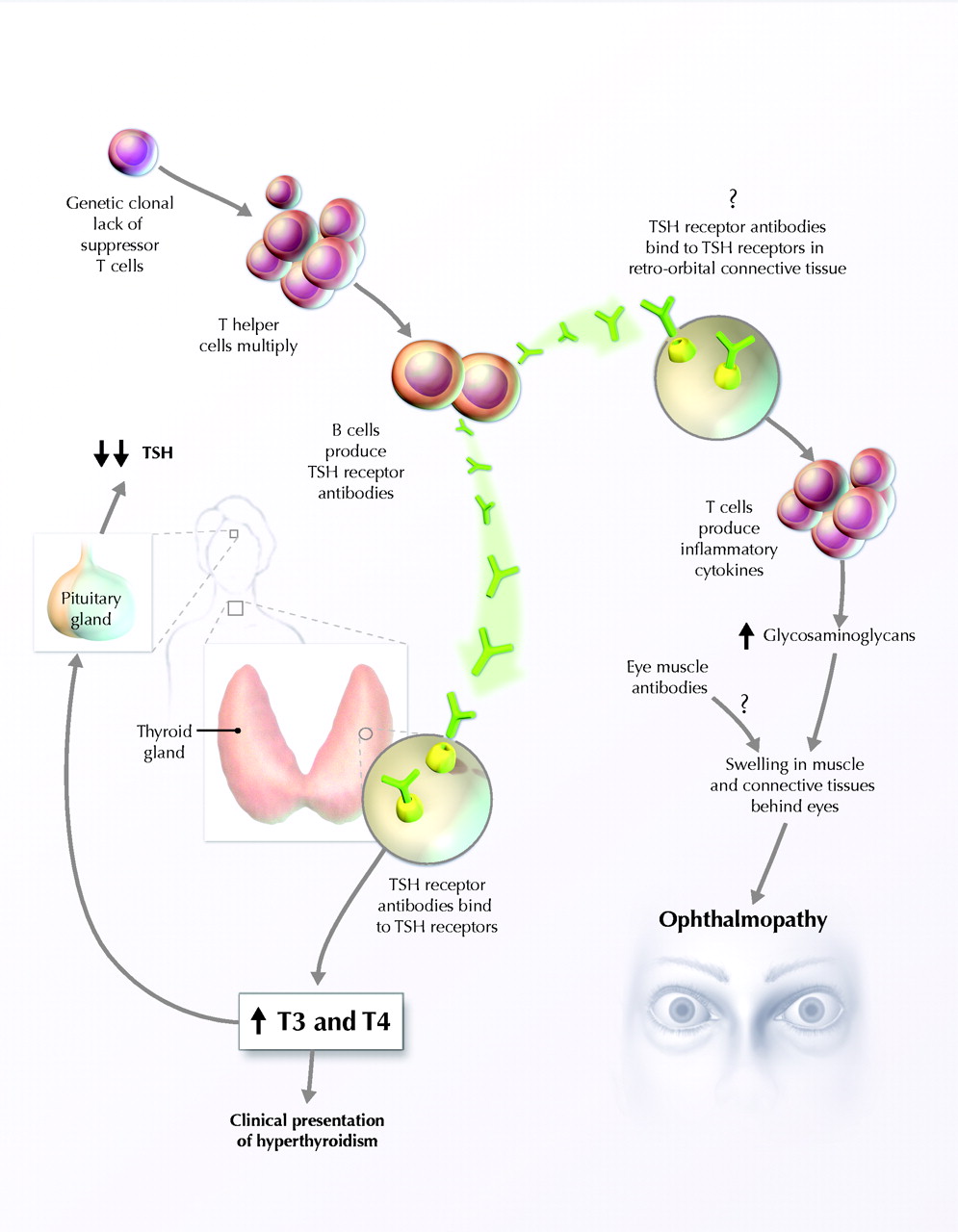
Figure 5: Proposed mechanism of Graves' disease pathogenesis.
Figure taken pending permission from Ginsberg,
2003.
One of the hallmarks of Graves' disease is the prevalence of a Th2 response rather than a Th1 or CD8 T cell response. The Th2 dominance is achieved through "abnormal" T cell-dependent B-cell activation, which leads to the release of cytokines specific for a Th2 response, particularly IL-10 (Itoh et al., 1998). Suppression of CD8 cells by this Th2 response may accentuate the symptoms of Graves' disease. CD8-expressing cells can work to counteract Th2 dominance; in fact, studies have shown that secretion of sCD8 is particularly marked during the active phase of Graves' disease (Watanabe, 1997). However, in hyperthyroid individuals, levels of CD8-expressing cells are lower than in euthyroid individuals, indicating that prevalence of the Th2 response is associated with more pronounced symptoms (Bossowski et al., 2003). A study by Aust, et al. (1996) suggests that NK cells, as well as CD8 cells, may help prevent B cell infiltration of the thyroid. In this study, some patients with high numbers of NK cells in their thyroid had lower levels of B cells than those with fewer NK cells.
Once they collect in the thyroid of Graves' disease patients, autoimmune lymphocytes express different molecules than their peripheral blood counterparts. Higher levels of CD8 are expressed and secreted by lymphocytes within the thyroid of affected individuals than in peripheral blood mononuclear cells (Itoh et al., 2002; Uchimura et al., 2002). The expression of cytokines changes as well. A study by Aust, et al. (2002) compared the expression of CXCR3 and CCR5 in peripheral and thyroid-derived lymphocytes in Graves' disease patients. They found that thyroid-derived lymphocytes expressed much more CCR5 and CXCR3 than lymphocytes from the peripheral blood of the same individuals. CXCR3 and CCR5 may be involved in recruiting more lymphocytes to the thyroid.
Apoptosis is abnormally regulated during the course of Graves' disease, and thyrocytes in affected individuals undergo lower-than-normal levels of apoptosis. These thyrocytes express subnormal levels of Fas and high levels of Bcl-2 (an anti-apoptotic protein). Conversely, thyroid-infiltrating lymphocytes (TILs) in Graves' disease express high levels of both Fas and FasL and low levels of Bcl-2 ; TILs in Graves' disease patients thus exhibit high levels of apoptosis (Salmaso et al., 2002). This abnormal apoptotic regulation suppresses the effects of infiltrating lymphocytes while allowing thyroid cells to persist longer than normal. These results contribute to hyperthyroidism.
Rather than targeting the immune response that causes Graves' disease, current Graves' disease treatments focus on restoring normal thyroid function by compensating for the hyperthyroidism of the disease. This is done either by radioiodine, antithyroid drugs, or surgery. The prevalence of each of these treatments varies regionally; each can be effective but also has potential side effects.
Radioiodine is the most common treatment for Graves' disease in North America. Patients ingest capsules or solutions of radioactive iodine, which is absorbed by the thyroid and causes localized death of thyroid tissue, typically counteracting hyperthyroidism within 6-18 weeks. The risk due to radioactivity is low--treatment has not been associated with "birth defects, infertility or overall cancer incidence" (Ginsberg, 2003). However, patients who are pregnant should not undergo radioiodine treatment because it could lead to hypothyroidism in their infants. A potential side effect of this and other treatments is the development of hypothyroidism in the patient as well. Conversely, some patients require more than one round of radioiodine to correct their hyperthyroidism.
Antithyroid drugs act by interfering with thyroid hormone synthesis. In patients with pronounced hyperthyroidism, treatment with antithyroid drugs may not be enough to send the disease into permanent remission, but 30 to 40 percent of patients treated with them undergo remissions of ten years or more (Weetman, 2000). For patients with a large goiter, thyroidectomy is often preferred because of its high efficacy in restoring euthyroidism, although hypothyroidism can result when most of the thyroid is removed. Hypothyroidism must be treated with supplementary thyroid hormones.
Graves' disease patients often experience temporary remission of their symptoms during pregnancy, a phenomenon that has sharpened our understanding of how autoimmunity is normally sustained in this disease. During the course of a normal pregnancy, the immune system changes to avoid rejection of the genetically and immunologically distinct fetus. Although conflicting data have been presented, a study by Kung and Jones (1998) indicated that pregnant women with Graves' disease experienced a decrease in the level of all types of T cells as well as NK cells throughout their pregnancies. However, the B cell levels did not decline, and the number of CD5+ B cells increased. Kung and Jones postulated that with pregnancy comes a "change in specificity from stimulatory to blocking antibodies" in Graves' disease patients. After pregnancy, symptoms of the disease usually return if the mother is untreated.
Unfortunately, Graves' disease can be vertically transmitted from mother to fetus, and approximately 1 to 5 percent of infants born to women with the disease are hyperthyroid. This is because IgG autoantibodies produced by the mother can cross the placental barrier and target the TSHR-expressing thyrocytes of the infant. Hyperthyroidism in fetuses is associated with poor growth and a high heart rate (Weetman, 2000). Once infants are born, their levels of maternal IgG decrease over time and eventually the symptoms of Graves' disease also cease. Hyperthyroid neonates can be treated with plasmapheresis, or replacing the plasma containing antibodies with normal plasma (Janeway, 2001).
Aust G, Sittig D, Steinert M, Lamesch P, Lohmann T. 2002. Graves' disease is
associated with an altered CXCR3 and CCR5 expression in thyroid-derived compared
to peripheral blood lymphocytes. Clin Exp Immunol 127(3):479-85.
Aust G, Lehmann I, Heberling HJ. 1996. Different immunophenotype and autoantibody
production by peripheral blood and thyroid-derived lymphocytes in patients with
Graves' disease. Exp Clin Endocrinol Diabetes 104(1):50-8.
Bartalena L, Marcocci C, Tanda ML, Manetti L, Dell'Unto E, Bartolomei MP, Nardi
M, Martino E, Pinchera A. 1998. Cigarette smoking and treatment outcomes in
Graves ophthalmopathy. Ann Intern Med 129(8):632-5.
Bossowski A, Urban M, Stasiak-Barmuta A. 2003. Analysis of changes in the percentage
of B (CD19) and T (CD3) lymphocytes, subsets CD4, CD8 and their memory (CD45RO),
and naive (CD45RA) T cells in children with immune and non-immune thyroid diseases.
J Pediatr Endocrinol Metab 16(1):63-70.
DeGroot L. 2003. The Thyroid and its Diseases. www.thyroidmanager.org. Accessed 2003 25 Apr.
Ginsberg J. 2003. Diagnosis and management of Graves' disease. CMAJ 168(5).
http://www.cmaj.ca/cgi/content/full/168/5/575.
Accessed 2003 8 April.
Giordano C, Richiusa P, Bagnasco M, Pizzolanti G, Di Blasi F, Sbriglia MS, Mattina
A, Pesce G, Montagna P, Capone F, Misiano G, Scorsone A, Pugliese A, Galluzzo
A. 2001. Differential regulation of Fas-mediated apoptosis in both thyrocyte
and lymphocyte cellular compartments correlates with opposite phenotypic manifestations
of autoimmune thyroid disease. Thyroid 11(3):233-44.
Heward JM, Allahabadia A, Daykin J, Carr-Smith J, Daly A, Armitage M, Dodson
PM, Sheppard MC, Barnett AH, Franklyn JA, Gough SCL. 1998. Linkage disequilibrium
between the human leukocyte antigen class II region of the major histocompatibility
complex and Graves' disease: replication using a population case control and
family-based study. J Clin Endocrinol Metab 83(10):3394-3397.
Holmes N. 2001. Immunology part IB home page. Department of Pathology, University of Cambridge. http://www-immuno.path.cam.ac.uk/%7Eimmuno/part1.html. Accessed 2003 25 Apr.
Itoh M, Uchimura K, Yamamoto K, Makino M, Imamura S, Kobayashi T, Fujiwara K,
Kato T, Hayakawa N, Sawai Y, Nagasaka A, Iwase K, Nomura T, Hagino Y. 2002.
Distinctive response of thyroid-infiltrating mononuclear cells to B cell activation
through CD40 and interleukin-4 in Graves' patients. Cytokine 19(3):107-14.
Itoh M, Uchimura K, Hayakawa N, Makino M, Hayashi R, Nagata M, Kakizawa H, Nagasaka
A, Sakamoto H, Kuzuya H. 1998. Surface expression and release of soluble forms
of CD8 and CD23 in CD40- and IL-4-activated mononuclear cells from patients
with Graves' disease (GD). Clin Exp Immunol 113(2):309-14.
Janeway C, Travers P, Walport M, Shlomchik M. 2001. Immunobiology: The Immune
System in Health and Disease. NY: Garland Publishing, 2001.
Kajava A. 1998. ISREC molecular modeling activities. http://www.isrec.isb-sib.ch/modeling/index.html. Accessed 2003 25 Apr.
Kung AWC, Jones BM. 1998. A change from stimulatory to blocking antibody activity
in Graves' disease during pregnancy. J Clin Endocrinol Metab 83(2):514-518.
Pani MA, Regulla K, Segni M, Hofmann S, Hufner M, Pasquino AM, Usadel KH, Badenhoop
K. 2002. A polymorphism within the vitamin D-binding protein gene is associated
with Graves' disease but not with Hashimoto's thyroiditis. J Clin Endocrinol
Metab 87(6):2564-7.
Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. 1998. The thyrotropin (TSH)-releasing
hormone receptor: interaction with TSH and autoantibodies. End Rev 19(6):673-716.
Salmaso C, Bagnasco M, Pesce G, Montagna P, Brizzolara R, Altrinetti V, Richiusa
P, Galluzzo A, Giordano C. 2002. Regulation of apoptosis in endocrine autoimmunity:
insights from Hashimoto's thyroiditis and Graves' disease. Ann N Y Acad Sci
966:496-501.
Thyroid Imaging World Wide Web. 2002. www.thyroidimaging.com. Accessed 2003 25 Apr.
Tsuyoshi K, Yoshikuni S, Gardine CA, Fisfalen ME, Alegre ML, DeGRoot LJ. 2000.
CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function
of CTLA-4 and contributes to the pathogenesis of Graves' disease. J Immunol
165:6606-6611.
Uchimura K, Itoh M, Yamamoto K, Imamura S, Makino M, Kato T, Fujiwara K, Saway
Y. 2002. The effects of CD40- and interleukin (IL-4)-activated CD23+ cells on
the production of IL-10 by mononuclear cells in Graves' disease: the role of
CD8+ cells. Clin Exp Immunol 128(2):308-12.
Watanabe M, Amino N, Hochito K, Watanabe K, Kuma K, Iwatani Y. 1997. Opposite
changes in serum soluble CD8 in patients at the active stages of Graves' and
Hashimoto's diseases. Thyroid 7(5):743-7.
Weetman A. 2000. Graves' disease. NEJM 343(17):1236-1248.
This page was produced by Melissa Breedlove.
Send any comments to mebreedlove@davidson.edu