This web page was produced as an assignment for an undergraduate
course at Davidson College.
Molecular Tool: Electroporation
Electroporation is a mechanical method used to introduce polar molecules into a host cell through the cell membrane. In this procedure, a large electric pulse temporarily disturbs the phospholipid bilayer, allowing molecules like DNA to pass into the cell (Purves et. al., 2001).
Background
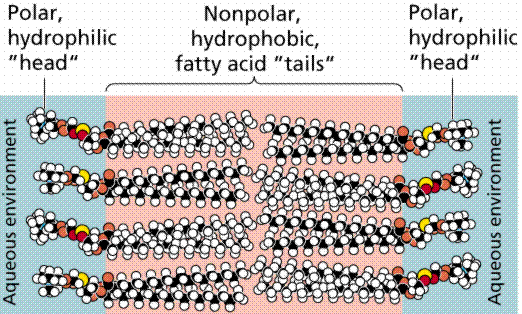
Many research techniques in molecular biology require a foreign gene or protein material to be inserted into a host cell. Since the phospholipid bilayer of the plasma membrane has a hydrophobic exterior and a hydrophobic interior (Fig. 1), any polar molecules, including DNA and protein, are unable to freely pass through the membrane (Farabee, 2001).

Figure 1. Diagram of the Phospholipid Bilayer. This image shows the chemical components of the plasma membrane. The polar head groups face outward while the hydrophobic tail groups face inward and interact with one another to hold the membrane together. Polar molecules cannot pass through this membrane without external aid (Farabee, 2001). Image from http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBooktransp.html Permission Pending.
Many methods have been developed to surpass this barrier and allow the insertion of DNA and other molecules into the cells to be studied. One such method is electroporation.
The concept of electroporation capitalizes on the relatively weak nature of the phospholipid bilayer's hydrophobic/hydrophilic interactions and its ability to spontaneously reassemble after disturbance (Purves, et. al., 2001). Thus, a quick voltage shock may disrupt areas of the membrane temporarily, allowing polar molecules to pass, but then the membrane may reseal quickly and leave the cell intact.
Procedure
The host cells and the molecules to be inserted into these cells are suspended in solution. The electroporation apparatus is typically commercially produced and purchased, but the basic process inside such an apparatus may be represented in a schematic diagram (Fig 2).
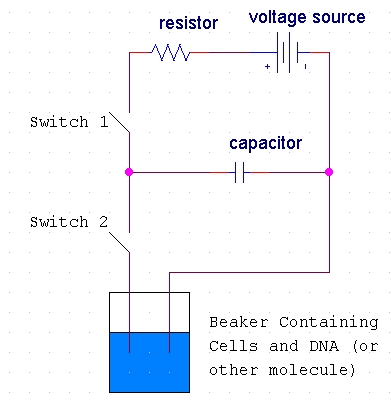
Figure 2. Diagram of the basic circuit setup of the electroporation apparatus. This diagram shows the basic electric circuit that provides the voltage for electroporation. Diagram idea from http://opbs.okstate.edu/~melcher/MG/MGW4/MG431.html
When the first switch is closed, the capacitor charges up and stores a high voltage. When the second switch is closed, this voltage discharges through the liquid of the cell suspension (Melcher, 2000). Typically, 10,000-100,000 V/cm (varying with cell size) in a pulse lasting a few microseconds to a millisecond is necessary for electroporation. This electric pulse disturbs the phospholipid bilayer of the membrane and causes the formation of temporary aqueous pores. The electric potential across the membrane of the cell simultaneously rises by about 0.5-1.0 V so that charged molecules (such as DNA) are driven across the membrane through the pores in a manner similar to electrophoresis (Fig 3).

Figure 3. Graphic representation of plasmids containing a foreign DNA insert passing through temporary aqueous pores in the plasma membrane. The actual entry of DNA into the cell cannot be observed with a microscope, but this artist's rendering shows the basic concept of the formation of pores in the membrane through which DNA can pass (Maxcyte, 2002). Image from http://www.maxcyte.com/technology.html Permission pending.
As charged ions and molecules flow through the pores, the cell membrane discharges and the pores quickly close, and the phospholipid bilayer reassembles (Weaver, 1995). The intended molecules should now be inside the cell for further use or study.
Advantages and Disadvantages of Electroporation
Several methods other than electroporation are used to transfer polar molecules like DNA into host cells. These other methods include microprecipitates, microinjection, liposomes, and biological vectors. (Melcher, 2000). Electroporation has both advantages and disadvantages compared to these methods.
Advantages:
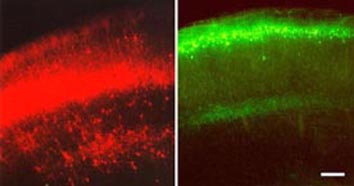
Figure 4. Image of in vivo electroporation in a mouse brain. The mouse brains (telencephalons) in these images are expressing reporter genes (EYFP) introduced in gene constructs by electroporation. Image from http://www.frontier.kyoto-u.ac.jp/rc01/in_vivo_electroporation.html Permission pending.
Disadvantages:
Applications
As previously mentioned, electroporation is widely used in many areas of molecular biology research and in the medical field. Some applications of electroporation include:
- DNA Transfection or Transformation: This is
likely the most widespread use of electroporation. Specific genes can be cloned
into a plasmid and then this plasmid introduced into host cells (bacterial or
otherwise) in order to investigate gene and protein structure and function.
(Nickoloff, 1995)
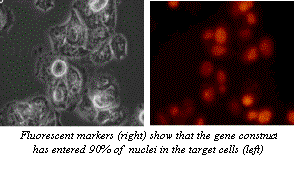
Figure 5. Microscope images of the results of transfection by electroporation. In this experiment, a gene construct was inserted by electroporation into the cells shown on the right. The fluorescence of the protein produced by the reporter gene included in this construct shows that the DNA was properly uptaken in the majority of cells. These cells could now be used in further experimentation (MaxCyte, 2002). Image from http://www.maxcyte.com/technology.html Permission pending.
- Direct Transfer of Plasmids Between Cells: Bacterial cells already containing a plasmid may be incubated with another strain that does not contain plasmids but that has some other desireable feature. The voltage of electroporation will create pores, allowing some plasmids to exit one cell and enter another. The desired cells may then be selected by antibiotic resistance or another similar method (Withers, 1995). This type of transfer may also be performed between species. Thus, large numbers of plasmids may be grown in rapidly multiplying bacterial colonies and then transferred to yeast cells by electroporation for study (Gunn et. al., 1995).
- Induced Cell Fusion: The disruption of the membrane that occurs with the quick pulse of electricity in the electroporation procedure has also been shown to induce fusion of cells (Weber and Berg, 1995).
- Trans-dermal Drug Delivery: Just as electroporation causes temporary pores to form in plasma membranes, studies suggest that similar pores form in lipid bilayers of the stratum corneum- the outermost dead layer of skin. These pores could allow drugs to pass through to the skin to a target tissue. This method of drug delivery would be more pleasant than injection for the patient (not requiring a needle) and could avoid the problems of improper absorption or degradation of oral medication in the digestive system (Praustnitz et. al., 1993).
- Cancer Tumor Electrochemotherapy: Scientists are investigating the potential of electroporation to increase the effectiveness of chemotherapy. As in electroporation for DNA transfection, the applied electrical pulse would disrupt the membrane of the tumor cell and increase the amount of drug delivered to the site. Some studies have suggested that increased tumor reduction is seen when this method is applied to cancerous cells in animal model systems (Maeda et. al., 1998).
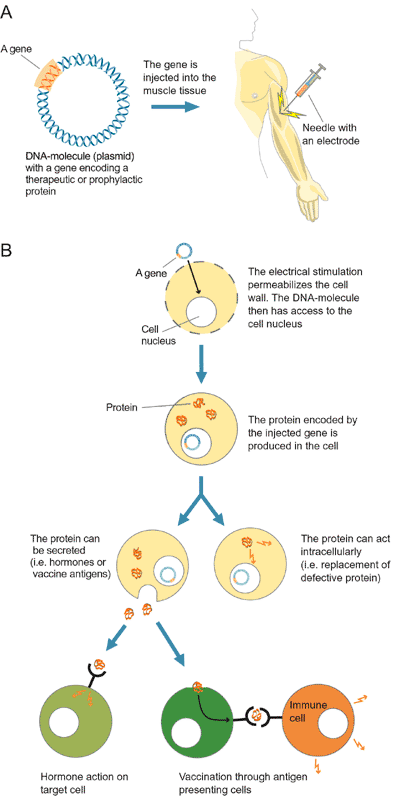
- Gene Therapy: Much like drug delivery, electroporation
techniques can allow vectors containing important genes to be transported across
the skin and into the target tissue. Once incorporated into the cells of the
body, the protein produced from this gene could replace a defective one and
thus treat a genetic disorder (Fig. 6) (Inovio, 2002).
Links
Molecular Genetics: Electroporation - This website, designed by a professor for a molecular genetics course, gives general information about electroporation and links to descriptions of other methods for inserting DNA into cells.
Transformation of E.coli by Electroporation - This website gives an example of a specific protocol used to transform bacteria by electroporation.
Electroporation System - This site markets an electroporation setup that can be purchased by molecular biologists and lists applications of such an apparatus.
Characterization of Cell Membrane Electroporation - This site gives more technical detail on the specific processes of electroporation.
References
Farabee, MJ. 2001. Transport In and Out of Cells. <http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBooktransp.html> Accessed 2003 17 Feb.
Gunn L et. al. 1995. Transfer of Episomal and Integrated Plasmids from Saccharomyces cerevisiae to Escherichia coli by electroporation. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press. p 55-66.
Inovio. 2002. Technology Platform: Electroporation. <http://www.inovio.com/technology_electroporation.shtml> Accessed 2003 15 Feb.
Maeda, H et. al. 1998. Electrochemotherapy Potentiation of Antitumor Effect of Cyclophosphamide by Local Electric Pulses on the Metastatic Lesion of Hamster Oral Fibrosarcoma. <http://www.mcmaster.ca/inabis98/cancer/maeda0671/index.html> Accessed 2003 15 Feb.
Maxcyte. 2002. Technology. <http://www.maxcyte.com/technology.html> Accessed 2003 15 Feb.
Melcher, Ulrich. 1997-2000. Molecular Genetics: Electroporation. <http://opbs.okstate.edu/~melcher/MG/MGW4/MG431.html> Accessed 2003 13 Feb.
Miller EM and Nickoloff JA. 1995. Escherichia coli Electrotransformation. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press. p 105-114.
Nickoloff JA. 1995. Preface. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press. p v-vi.
Prausnitz MR et al. 1993. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci USA 90: 10504-8.
Purves WK. et. al. 2001. Life: The Science of Biology- 6th ed. Sinauer Associates, pp.316-317.
Saito, Tetsuichiro. 2001. In Vivo Electroporation. <http://www.frontier.kyoto-u.ac.jp/rc01/in_vivo_electroporation.html> Accessed 2003 15 Feb.
Weaver JC. 1995. Electroporation Theory: Concepts and Mechanisms. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press. p 1-26.
Withers HL. 1995. Direct Plasmid Transfer Between Bacterial Species and Electrocuring. In: Nickoloff JA, editor. Electroporation Protocols for Microorganisms. Totowa, New Jersey: Humana Press. p 47-54.
Questions or Comments? Please e-mail Rachel Patton McCord
Rachel Patton McCord's Molecular Homepage
Davidson College Molecular Biology