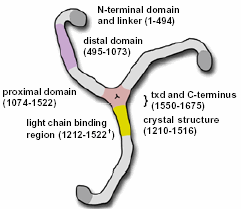
This is a chime diagram of the proximal domain of one leg of clathrin.
Purpose
The purpose of this page is to describe a my favorite protein, Clathrin, by its cellular component, biological process, structure and biological function. In addition I will also show orthologs of this protein among different species as well as mutant derivatives of this protein.
Cellular Component
Clathrin is found free floating in the cytoplasm and on the cytoplasmic side of intracellular organelles which are refereed to as coated vesicles or coated " pits ". The gene for clathrin in humans is named CLTD and its chromosomal location is 22q11.2 (OMIM).
Biological Process
The coated vesicles, or pits, are themselves involved in intracellular trafficking of protein receptors and endocytosis of macromolecules. Clathrin too is intimately involved in the very same processes because clathrin is always bound to these vesicles by interacting with the cytoplasmic tails of integral membrane proteins that are within the lipid membrane of the vesicles (OMIM). Thus the biological process of clathrin is trafficking of protein receptors and endocytosis of macromolecules.
Structure and Function of Clathrin
The structure of clathrin is described as a " triskelion " which comprises of three heavy chain legs. Each one of the legs is 1675 aa long and has a approximate weight of 192 kDa (Pearse, B et al). Each leg consists of four main domains; the N-terminal end ( 1-494 aa ) which is where the the clathrin legs harbor clathrin binding sites which bind to vesicles, the distal domain ( 495-1073 aa ) and proximal domains which are involved in associating with other clathrin proteins to form the coating lattice structure around vesicles, and the C-terminal end ( 1550-1675 aa ) which is where the legs of the clathrin join to form the central trimerization domain (txd).

This is a picture of the components of clathrin. Its displays the different domains of clathrin. Permission pending from fmarbro@ucsf.edu.
Clathrin forms what is called a polyhedral lattice around vesicles which is basically a soccer ball design. This is done by the proximal and distal domains of clathrin legs associating with one another ( Figure below )
.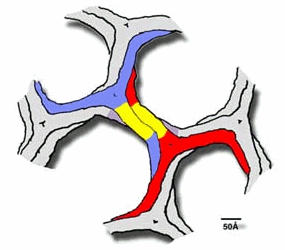
* Permission pending from http://www-als.lbl.gov/als/science/sci_archive/clathrin.html *
This polyhedral lattice forms an encapsulating coat of clathrins around the entirety of a vesicle and aids in the trafficking of the vesicle to its destination. The legs of clathrin are described as having seven repeating 10-helices known as clathrin heavy-chain repeats, CHCR's. ( Figure below )
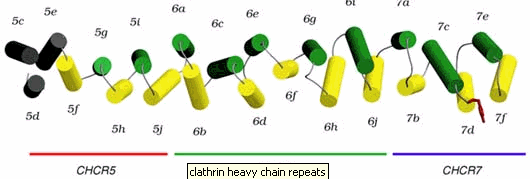
* Permission pending from http://www-als.lbl.gov/als/science/sci_archive/clathrin.html *
The helices are bound together by multiple hairpin turns and loops. The CHCR structure gains its strength by the hairpin turns and loops that hold it together (http://www-als.lbl.gov/als/science/sci_archive/clathrin.html). The structure of the clathrin requires strength because it needs to be able perform exocytosis as well as endocytosis which means it must be able to pinch away portions of plasma membrane to form a vesicle. For a great video that shows the formation of the polyherdal lattice go to : http://www.hms.harvard.edu/news/clathrin/clathrin_big.html . Below is a picture of actual clathrin triskelions that have made polyhedral lattices. This picture was taken with a electron microscopeby the Heuser Lab.
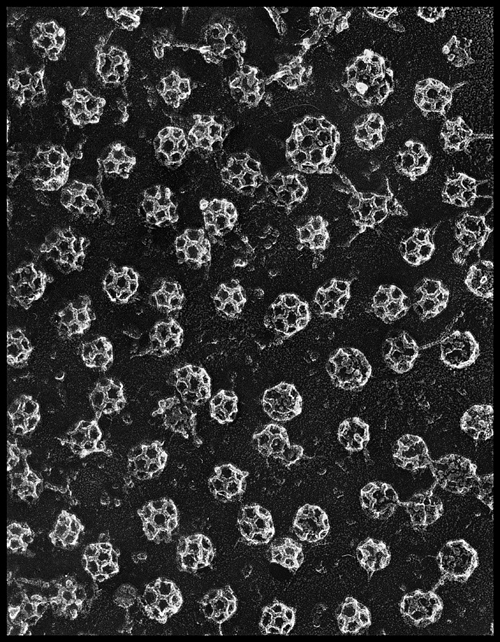
* Permission pending from http://www.heuserlab.wustl.edu/v2.0/index.shtml *
Clathrin Orthologs and Mutations
Clathrin is found in the cells of humans, mice, Arabidopsis thaliana and hypothetically in slime mold. Mutations in the clathrin sequence that lead to mutant clathrin proteins can result in DiGeorge syndrome which causes facial dysmorphia, mental retardation, long slender digits and genital abnormalities (OMIM).
Conclusion
Clathrin is a necessary protein for proper intracellular vesicle transport. Its very unique structure is tailored to efficiently performing endocytosis of other proteins by extracting them from the plasma membrane of intracellular organelles to form coated pits which carry the encapsulated proteins to their destinations. Mutations in the CLTD coding region of clathrin in the genome lead to severe health problems in humans and is not conducive to a viable organism. Finally, clathrin is found across many species and is clearly a most fit protein for its function.
References and Links Used
" Clathrin Structure Reveals Motifs for Self Assembly " The Advanced Light Source, Accessed March 12, 2003. http://www-als.lbl.gov/als/science/sci_archive/clathrin.html
" Clathrin Coat Construction in Endocytosis " Pearse, Barbra et al, Accessed : March 12, 2003. http://www.carleton.ca/~jcheetha/macro/seminars/clathrin.pdf
" Sequential Steps in Clathrin Mediated Synaptic vesicle Endocytosis " Brodin, Lenart et al, Accessed March 12, 2003. http://www.carleton.ca/biochem/macro/readings/endocytosis.pdf
The Protein Database http://www.rcsb.org/pdb/index.html
OMIM http://www3.ncbi.nlm.nih.gov/Omim/searchomim.html
NCBI http://www.ncbi.nlm.nih.gov/
Swiss Prot http://us.expasy.org/sprot/
These web pages were produced as an assignment for the undergraduate Biology 304 : Molecular Biology course at Davidson College.
If there are any questions concerning this website please e-mail me : patoran@davidson.edu