Review Paper
The immunoglobin superfamily protein Izumo is required for sperm to fuse with eggs
Naokazu Inoue, Msahito Ikawa, Ayako Isotani, and Masaru Okabe
Nature, Vol. 434, 234-238, 10 March 2005
Reviewed by Kelly Dresser, April 2005
Background to better understand the Izumo protein:
Normal fusion of sperm and egg process
*This paper proves that a mutation in Izumo creates the inability for the sperm to fuse to the egg and undergo this acrosome reaction.*
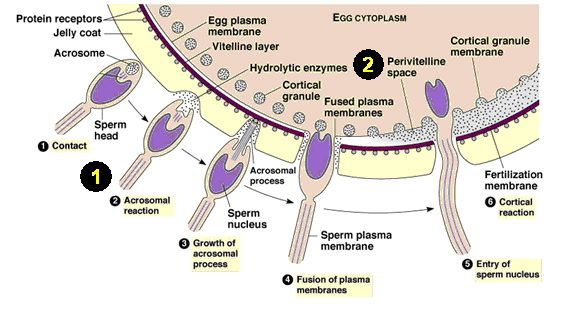
Image 1. A diagram of sperm and egg fusion in the fertilization process. Labels 1 and 2 are defined below.
http://web2.uwindsor.ca/courses/biology/weis/55-101/lec16a.ppt (see slide 5)
1. The acrosome reaction: An acrosome reaction is the process of fertilization, just prior to penetration into the egg, when the sperm is activated. In this reaction, enzymes are often released and surface antigens are exposed as a portion of the cell ruptures, which allows the sperm to "perform" (Shared Journey, 2003).
2. The perivitelline space: This area is the fluid-filled area between the egg and yolk membranes. Sperm must undergo the acrosome reaction in order for it to enter this section of the egg to eventually be fertilized. (Figure 3.d. shows the importance of this area later.)
Izumo is a type I membrane protein. This image may be what Izumo looks like within a sperm cell.
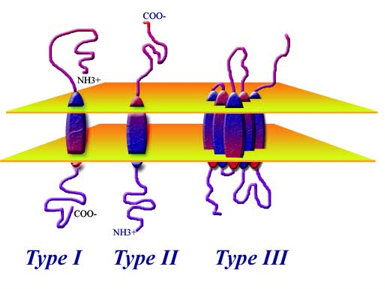
Image 2. The figure on the left is what the Izumo protein may look like within the plasma membrane of a sperm cell. Type II and III are other types, but do not pertain to this experiment.
http://www.proteinchemist.com/mem/mem.html
Summarized Results:
It has been found recently that CD9 on the egg membrane is essential for fusion, but this research team has designed an experiment to find other sperm-related fusion factors that remain unknown. By using a fusion-inhibiting monoclonal antibody and gene cloning, they found a fusion-related antigen in the mouse sperm to study further. The monoclonal antibody that was created and used was OBF13, and it worked to inhibit the fusion process of mouse sperm. The antigen that was identified was a novel immunoglobulin superfamily protein, which they named "Izumo". When this gene is mutated the mice remained healthy, but the males remained sterile regardless of what type of female they mated with. Not only do mice contain this Izumo gene, but humans as well. It was thought and later proven by this paper that this Izumo gene is responsible for the fusing of the sperm and the egg in fertilization. The following review of this paper gives analysis on the figures through out this paper and their validity to this experiment. There are positive and negative aspects to this study on the Izumo gene and its affects. Because of their accomplishments in proving their findings, there are also more experiments which could be done to improve and expand their conclusions about this particular immunoglobulin superfamily protein.
Figure 1.a. is a comparison of the amino-acid sequences of the mouse and human Izumo gene. The mouse sequence is on the top and the human sequence is on the bottom. The asterisks under the two sequences represent where the amino-acid sequences of the two species are identical. The red highlighted amino acids are peptide sequences that have been obtained by liquid chromatography tandem mass spectrometry (LC-MS/MS). The putative signal peptide region is shown in orange and the transmembrane region is shown in blue. The green box is the immunoglobulin-like domain. The two arrow heads point out the cysteine residues that might form disulfide bridges with each other. This is a simple comparison of the mouse and human genes to see similarities in order to study both species.
Figure 1.b. is a cartoon of the Izumo gene. This image shows Izumo as a typical type I membrane glycoprotein with a disulfide bond, which was pointed-out in Figure 1.a. A type I membrane protein has a single transmembrane (TM) stretch of hydrophobic residues, with the portion of the polypeptide on the NH2-terminal side of the transmembrane domain exposed on the exterior (amino terminus) and the COOH-terminal portion exposed to the cytoplasmic side (carboxyl terminus) (Carter, 2003). (See Image 2 to visualize a type 1 membrane protein)There is also one immunoglobulin-like domain on this protein, which is demonstrated by the extension off the loop. This structure also contains a putative N-glycoside link motif within the immunoglobulin-like domain where a sugar is attached to an NH2 group off the amino acid, asparagine (Asn) 204. This image is affective for seeing the protein in cartoon form.
Figure 1.c. is a western blot that has been probed with the anti-mouse Izumo antibody. Each column tested a different tissue within the mouse and the only two that showed expression with the Izumo protein were the last two lanes, the testes and sperm, respectively. The only problem with this figure is the lack of a control. It says there was the same amount of protein loaded (30 ug) in each lane, but it cannot be trusted without physical evidence. It does provide useful information, though, to see that the protein is testes and sperm specific.
Figure 1.d. is also a western blot, but of the human Izumo protein, rather than of a mouse. The blot only shows the sperm lane that was ran and the arrow specifically points out the location of this protein of interest. However, the blot is useful in demonstrating the presence of this Izumo protein in human sperm cells. From this data we can hope for the possibility to work with mouse and human genes to study mutations of the Izumo protein and its effects. Even with positive results in the favor of the experiment, it is hard to accept this data as complete truth because there are no other lanes shown to compare the presence of this band. Also, it is interesting to note that the molecular weight of the Izumo protein in the human sperm is lower than that of the protein in the mouse sperm.
Figure 1.e. and 1.f. are images of immunostaining of Izumo in sperm from an acrosin-promoter-driven transgenic mouse (1.e) and human (1.f.) line that has been probed by green fluorescent protein (GFP). It has been enhanced by GFP in the acrosome. The acrosome is a protrusion on the anterior end of a sperm cell containing digestive enzymes that enable the sperm to penetrate layers around the oocyte (Lefers, 2004). In Figure 1.e., all three slides are of fresh sperm with intact acrosomes, but have been dyed with GFP to see where Izumo is expressed in different places. In the top slide and the bottom slide, the green arrows indicate that the Izumo protein was not detected in the sperm. In the second slide, the non-green fluorescent sperm, which has been stained red, shows that Izumo is present on acrosome-reacted sperm when stained with polyclonal antibody against the mouse Izumo (indicated by white arrow heads). In figure 1.f. of the human sperm, the sperm are stained green by anti-CD46 antibody instead of GFP and were reactive to the antibody against human Izumo, but did not react to acrosome-intact sperm. This data which shows the absence of the Izumo protein in both 1.e. and 1.f. are concurrent with the fact that fertilization is only possible after an acrosome reaction. To learn how the acrosome reaction works to fuse the sperm and egg, see Image 1: "Normal fusion of sperm and egg process". From the figure 1.f., we see that only after an acrosome reaction, did Izumo become detectable. This may mean that the Izumo protein is located under the plasma membrane and upon the acrosome reaction, the protein becomes exposed, so then Izumo is detectable. (See Image 1. at top - the error is caused
Figure 2 focuses on the disrupting of the Izumo gene to see the physiological role is plays. Figure 2.a. is a restriction structure map of the wild-type mouse Izumo allele, the targeting vector, and the mutant allele with the inserted neomycin resistant gene (neo r ) and a diphtheria toxin A chain (DT). By this forced mutation, a Izumo-deficient mouse has been made by homologous recombination. The horizontal bars in the image are introns; where as the shorter vertical bars are exons. In order to insert neo r and DT, exons 2-10 were replaced. These mutations have been done to disrupt the Izumo gene and see physiological differences in the mice.
Figure 2.b. is a southern blot that was conducted to confirm the gene disruption illustrated in Figure 2.a. The lanes are wild-type, heterozygous, and homozygous mutant mice, respectively. On the right side, the arrows point to the wild-type (EcoRI-digested genomic DNA yielded 15 kb) and the mutant (6.9 kb) bands. The data in this blot seems rather blurry, but from the darker, more predominant bands it seems that the wild-type bands are logical, and the mutant bands seem to line-up with the heterozygous and homozygous mice. This may confirm that the targeted genes (Izumo) are part of germline transmission.
Figure 2.c. is a northern blot, showing how the total testis RNA of wild-type, heterozygous, and homozygous mice are affected. GAPDH in the lower panel is the loading, positive control. The top panel shows the expected information, that the homozygous mice for the mutated allele, does not transcribe the Izumo RNA. Figure 2.d. shows similar data. In this figure, a western blot has been conducted to demonstrate that again the Izumo protein is not detected in the homozygous mutant mice. To confirm this result, three other related genes that are involved in sperm-egg interactions were tested. A positive control was added with ADAM2, showing full expression in each lane. In addition, CD147 and sp56 genes act as controls because they too show expression of their gene in all lanes, even the mutated version. These blots were very efficient in demonstrating the data needed to prove that the Izumo gene, RNA, and protein are not expressed when mutated in mice.
For these set of figures, intercrosses were made between the heterozygous F1 mice and their offspring were evaluated statistically. The data shows the infertility of males when the Izumo gene is disrupted. From Figure 3.a., fecundity of Izumo +\- , Izumo -\- males, and Izumo -\- females are tested. Fecundity is basically the potential reproductive capacity of an organism or population, measured by the number of gametes (offspring in this experiment) (McLeod, 1996). This graph shows that Izumo -\- females with heterozygous Izumo +\- males, demonstrated normal fecundity, but Izumo -\- males with Izumo +\+ females did not show normal fecundity - they were sterile. This shows that the homozygous mutated version of the Izumo gene only affects a mouse developmentally if they are a male. A male is not affected if he has Izumo +\- and likewise does not affect the female. In addition, if the female has a mutated Izumo gene, she does not affect offspring production.
Figures 3.b. and 3.c. are conducted by in vitro fertilization. The graph in Figure 3.b. shows that Izumo +\- sperm has almost 100% pronucleus formation, but sperm of Izumo -\- mice have no pronucleus formation (or an absence of zona-reaction which does not allow the sperm to bind to the zona pellucida). Figure 3.c. demonstrates this phenomenon in a captured image. It is obvious that in the bottom panel, the sperm have not entered the zona pellucida (the egg) and remain on the periphery, unable to fuse into the egg.
Figure 3.d. the upper panel shows the accumulation of sperm in the perivitelline space (see Image 1, label #2) of the eggs in the females recovered that mated with Izumo -\- males. By sperm entering this area, it shows that Izumo -\- does not cause an error in zona penetration, but in a step following penetration into the zona pellucida within the perivitelline space of the eggs. The lower panel is labeled with acrosome-reacted, sperm-specific monoclonal antibody MN9, further confirming that sperm is expressed with Izumo -\- after the acrosome reaction, but in this image the sperm has still failed to fuse with the eggs.
The graph in Figure 3.e. is of the average number of fused sperm observed in 2 and 6 hour intervals after insemination. Again, there is evidence for sterility in the Izumo -\- sperm. This graph also expresses that more sperm fuse with the egg with more time and regardless the amount of time, Izumo -\- sperm will never fuse with the egg. Only upon the zona pellucida being mechanically removed, the Izumo -\- sperm will be capable of binding to the plasma membrane of the eggs. Figure 3.f. shows that if the zona pellucida is mechanically removed, that the sperm will bind. In the second column of images that have been stained with Hoechst 33342 (targets and stains the sperm heads), the sperm have seemed to fuse (shown by the arrowheads in Izumo +\- sperm). In the Izumo -\- sperm, stained with Hoechst 33342, there is nothing in the image to suggest fusing with the egg. The only question is the small blue dot that appears in the black background. That is a little questionable if possibly one sperm could have entered the zona pellucida. There was no explanation for the specific use of Hoechst 33342, so the absence of sperm fusing with the egg in Izumo -\- seems logical, but inconclusive. It is not exactly certain whether this is what was "supposed" to occur.
Table 1- For this table, the research scientists took fertilization into their own hands. They used intracytoplasmic sperm injection (ICSI) (a laboratory procedure developed to help those with infertility undergo in vitro fertilization by directly injecting a single sperm into the cytoplasm of a mature egg (Rebar, 2001)) to insert Izumo -\- directly into the wild-type eggs to avoid the fusion step that was continuously failing. The eggs were then fertilized and transplanted into oviducts of pseudo-pregnant females. Surprisingly, the embryos developed normally and appropriately and acted similarly to the heterozygous mice. The offspring that were born even possessed the Izumo -null allele, so they would not pass this mutation onto their offspring. From this data, it seems that the fusion of sperm and egg is truly affected by the mutation in the Izumo gene.
Figures 4.a. The research scientists were not satisfied yet with their data and took one more step to test the Izumo gene in a xeno-species fusion system. They also wanted to assess human sperm fertility under these conditions. In figure 4.a., zona-free hamster eggs were used with mouse Izumo +\- and Izumo -\- sperm. They saw that homozygous Izumo -\- sperm was essential to fusion with hamster eggs, as well as the heterozygous sperm. Again, a good copy of the Izumo gene in sperm is shown to be necessary for fusion with an egg.
Figure 4.b. is an attempt at human application to this study. They used the anti-human Izumo polyclonal antibody in the incubation mixture and again no fusion was observed. In the top panel, IgG was used as a positive control and sperm treated with IgG fused with the eggs. The bottom panel was treated with anti-human Izumo and shows no fusion again, even within the human sperm. If the top panel is the control and shows fusion, and the bottom does not, there should be a different in the two "phase" boxes, but no difference seems to be present. Compared to Figure 4.a. there is a difference in the top and bottom panels, where as 4.b. does not show this (the only difference is seen in the "Hoechst 33342" boxes). Because this image was stained with Hoechst 33342, the data does show that human Izumo is involved in the fertilization process in human sperm. This result then implies that more investigation will be necessary to explain the function of Izumo in human fertilization.
Critique:
The results definitely support the author's claims. Both the sequence of the research and its presentation in the paper was very well executed. The paper is convincing in its arguments because it easily laid out the steps necessary for the reader to follow the data and understand its conclusions. All the data seemed to go as planned.
In figure 1 they showed that the Izumo protein is expressed in the mouse testes and sperm and human sperm, as it should if Izumo is supposed to affect sperm fusion with a female egg. In 1.e. and 1.f. they were even able to show when that fertilization by sperm is only possible after an acrosome reaction, and the absence of Izumo prevented fertilization. Figure 2, in general, efficiently portrayed that the homozygous mutant Izumo allele is not expressed in DNA, RNA, or protein, but I found the quality of the blots to be lacking precision. They were quite blurry and Figure 2.b. in particular did not really show definite data. The blot seemed to be pretty smeared even though the bands appear darker in the places they "should be" darker. The most beneficial figure was figure 3. It was well planned to show the graph for the data along with two images that displayed the same information. It made the data much easier to follow and understand. Figure 3 visually showed how the disruption in the Izumo gene can affect male infertility. In addition, they further made progress to compare the homozygous Izumo to the heterozygous Izumo to show that by ICSI, the two types of sperm act the same way to fertilize and allow development of healthy offspring. Last, Figure 4 just clarified the previous data, so all corners were covered. They found that a "good version" of the Izumo gene is necessary for sperm fusion with an egg. One of the most valuable results was in figure 4.b., which showed that human Izumo is needed in the fertilization process in human sperm. Because of this piece of data, it is possible that these conclusions could result in further tests that may lead to new contraceptive strategies and answers to helping those with infertility.
One aspect that should have been addressed was the use of the zona-free hamster eggs. Are hamster eggs particularly useful in these types of experiments? And why would they be more useful that making a mouse have zona-free eggs? Also, I may have needed to know more about egg fertilization, but Figure 3.d. was supposed to show that the sperm penetrated the zona pellucida and that it was now in the perivitelline space. How could the sperm penetrate the zona pellucida, if it was impaired? It hadn't entered the egg previously, why now?
Over all I found the data adequate to support the claim that Izumo in humans and mice is essential for sperm fusion with an egg.
Future Experiments:
For future experiments, it will be interesting to see what developments can be made to make contraceptives based on this information. If a contraceptive can be taken by a man to inhibit the Izumo protein, then none of their sperm would fertilize an egg. With infertile patients, there may be medication in the future that could be taken or administered that activates Izumo in human sperm or adds the protein artificially so that fusion can occur.
More important experiments need to be done to better understand what other proteins may be responsible for fusion with an egg. The exact location of Izumo may be a factor to why it may be limiting the acrosome reaction, but this is not known at this time. To test if Izumo's location affects its behavior, they could transpose the protein to different parts of the sperm or see if similar proteins in different areas are affected similarly. I would assume that because they suspect this protein to be hidden under the plasma membrane and it only detectable upon the acrosome reaction, that location has a great affect on its behavior. Localization is thought to not be limited to the equatorial segments where fusion initially takes place, so with the absence of Izumo, the protein must change its location and enable the sperm nucleus to localize at the equatorial segments. To track the movement of these proteins, they could be labeled with different fluorescent proteins like GFP and followed through phases in the fertilization process. By a gradient, a protein could be analyzed in BLAST.
As mentioned in the beginning, CD9 has also been found to be essential in fusion. If they know how CD9 works with other proteins, then it would be helpful to know the interaction that Izumo may have with CD9. If they are working together, then one protein may be offsetting the other to homologously recombine and cause its inability to make sperm fuse.
On the outside of sperm there are galactosyl transferase (GalTase) enzymes on its head and ZP3 on the outside of an egg. The GalTase on the sperm surface acts as the receptor for ZP3 because it binds to galactose on ZP3. Upon this binding, the receptors on the sperm head cluster and increase levels of calcium in the sperm cell. "Calcium is a known mediator of biomembrane fusion and this increase of intracellular calcium plays a role in the fusion of the acrosomal membrane with the sperm cell membrane causing vesicles to form" (O'Day, 1998). Then, the membrane has fused and the acrosome reaction occurs, releasing the enzymes within the acrosome. First, it could be possible that this Izumo protein is inhibiting these receptors to bind to ZP3 on the egg, so calcium levels can increase and cause fusion. Experiments could be conducted to see the interaction between Izumo and GalTase or levels of galactose. Maybe the absence of Izumo stops a cascade of reactions to allow the sperm to open the receptors to the ZP3 on the egg. Furthermore, Izumo could be causing problems with calcium production. The binding could be occurring, but due to a lack of calcium produced or lack of vesicles forming, fusion is not possible. Levels of calcium could be tested by ionic measures. First, mutant homozygous, heterozygous, and wild-type sperm could be isolated. Sodium chloride could be added to this sperm and the chloride would bind to the Calcium, leaving sodium unbound. Then, the measure of sodium could be measured by a change in pH to see if the concentration of Calcium changed due to binding to the chloride ions. By measuring a change in pH within the sperm cells, levels of calcium could be collected.
By better understanding this cell-cell fusion process between the sperm and egg, maybe information concerning other cell-cell fusions will become available in the future. There are endless possibilities to where this novel piece of information can go.
Izumo essentially means "happy marriage" in Japanese and by being named after a Japanese shrine dedicated to marriage, if this Izumo protein is finally understood, it may cause a "happy marriage" not only between a couple if they can finally become fertile, but also between the scientists and their work (and money).
References:
Carter, Darrick, Ph.D. (2003). "Membrane protein topology". accessed 15 Apr 2005. http://www.proteinchemist.com/mem/mem.html
Lefers, Mark. (July 2004). "Acrosome".accessed 15 Apr 2005. http://www.biochem.northwestern.edu/holmgren/Glossary/Definitions/Def-A/acrosome.html
Shared Journey. (2003). "Acrosome Reaction". accessed 25 Apr 2005. http://sharedjourney.com/search.html (definition: acrosome reaction).
McLeod, C.R.. (1996). "Fecundity". MarLIN, The Marine Life Information Network for Britain and Ireland, accessed 25 Apr 2005. http://www.marlin.ac.uk/glossaries/fecundity.htm
Rebar, Robert W., MD. Director. (Aug 2001). "Intracytoplasmic Sperm Injection (ICSI)". American Society for Reproductive Medicine. accessed 4/15/05. http://www.asrm.org/Patients/topics/icsi.html (fifth bullet).
Danton H. O'Day. (1998). "Fertilization: Intercellular Communications & Signal Transduction". accessed 26 Apr 2005. http://www.erin.utoronto.ca/~w3bio380/lecture/Lect08/L8.htm
Davidson College Biology Home Page
© Copyright 2005 Department of Biology, Davidson College, Davidson, NC 28036
If you have any questions, comments, or suggestions concerning this page, please contact kedresser@davidson.edu