General Information
Aplastic anemia (AA) is a relatively rare disorder that occurs when the stem cells within the bone marrow cease their normal functioning and produce too few, or no blood cells at all. This includes all three types of blood cells: red blood cells, white blood cells, and platelets. The slowed or complete loss of production of blood cells can be detrimental to ones health, and this disease should be taken very seriously.
When red blood cells are produced in fewer numbers, hemoglobin levels can drop significantly, causing problems with oxygen delivery to various parts of the body and symptoms associated with normal anemia. When white blood cells are produced in fewer numbers, the immune system is affected in a negative way, and it decreases the body's ability to fight off infections, resulting in neutropenia. When platelets are produced in fewer numbers, blood is unable to clot as readily as it is in patients with normal platelet levels, resulting in thrombocytopenia, which may cause excess bruising and bleeding (Young 2002).
Cause
One common cause of AA is transient bone marrow failure that results from both chemotherapy and radiotherapy used to treat various forms of cancer, namely Leukemia (see Figure 1-B and C ) . The chemo and radio therapies damage the DNA of the hematopoietic cells within the bone marrow which causes them to undergo apoptosis, and ultimately, this results in a loss of function of the bone marrow (Young NS, et al 1997). The introduction of foreign chemical substances that have toxic effects which can damage bone marrow has also been shown to cause AA. For example, in some developing countries, it has been shown that benzene is linked with several cases of AA in Bangkok, Thailand (Issaragrisil S, et al 1991).
For a long time, however, medical professionals were confused as to what a cause of AA other than direct hematopoietic injury could be, but recently (within the past thirty years), there has been increased evidence to support the hypothesis that it is a direct result of an auto-immune reaction that targets the hematopoietic stem cells within the bone marrow (Kagan WA, et al 1976; Zoumbos, et al 1985). It has been shown that sometimes there can be stimuli that cause normal cellular peptides to be presented by MHC molecules, or stimuli that alter cellular peptides slightly so that they are recognized as foreign pathogens and are targeted by the immune system. One such stimulus is a viral infection (see Figure 1-A). In some very rare instances, when hematopoietic bone marrow cells are infected with a virus, they will present their own proteins to antigen presenting cells, which then allow naive T-cells to develop receptors which recognize these self proteins. These T-cells can then proliferate and attack the remaining hematopoietic bone marrow cells that have the same surface proteins as the ones that were originally infected with the virus (Young NS, et al 1997).
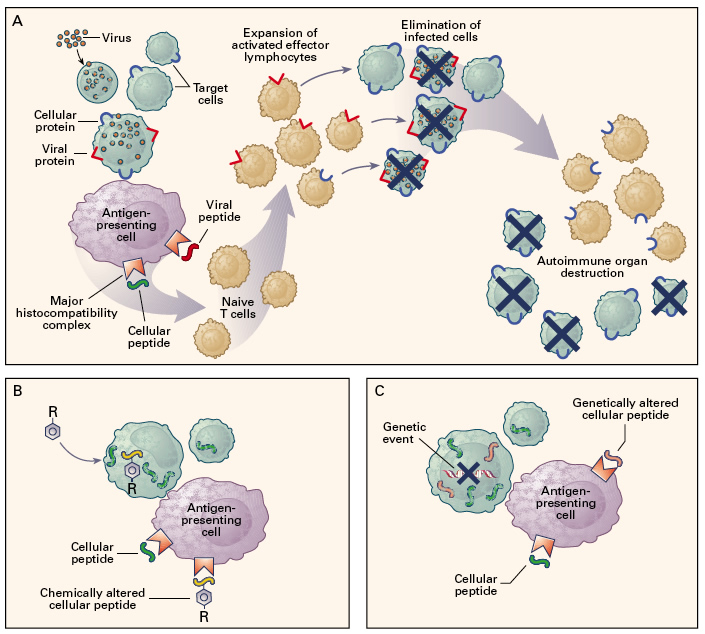
Figure 1. Various immune responses that result in the degradation of hematopoietic bone marrow cells. In panel A, hematopoietic cells (green) are infected with a virus which may sometimes lead to the presentation of both viral proteins and cellular proteins on the cell surface via MHC molecules. These cellular proteins may then be recognized by T lymphocytes which will terminate them because they are recognized as being harmful. The result is the loss of hematopoietic cells within the bone marrow, which causes AA. Panel B shows how cellular peptides are sometimes altered by chemicals, such as drugs. These altered peptides are then recognized by the immune system as foreign particles, and the cells that contain them then become targets of the immune system, again resulting in the destruction of hematopoietic bone marrow cells, which causes AA. Similarly, in Panel C, cellular peptides may be altered by some random or induced genetic mutation, which can cause them to be recognized as foreign by the immune system; again resulting in the degradation of hematopoietic bone marrow cells (Young NS, et al 1997) Permission Pending.
In addition to viruses, foreign substances such as drugs can also cause an autoimmune reaction that can result in the destruction of hematopoietic bone marrow cells, leading to AA (see Figure 1-B). Certain drugs can bind to surface proteins and alter them slightly, which causes them to be recognized as foreign antigens by the immune system. Any other cells presenting the altered surface protein will subsequently be attacked by the immune system, which can result in AA (Issaragrisil S, et al 1991).
Furthermore (as is shown in Figure 1-C), an autoimmune response that can result in AA can also occur as a result of genetic mutations, which can occur spontainiously, as a result of exposure, or from chemo or radio therapies for certain types of cancer. Immunologically, the response is the same as it is for the previously mentioned means of acquiring AA via an autoimmune response, but the method by which the self protein is altered is different. Instead of being influenced by a virus or by chemicals, the cellular protein is altered at the genetic level, or in the DNA. The result is a protein on the surface of the cell that is not recognized as self protein by the immune system. If this occurs in hematopoietic bone marrow cells, they may subsequently be targeted by the immune system, and AA may result (Young NS, et al 1997).
Diagnosis
Diagnosis of AA generally takes sometime. Seeing as how it is a rare disease, it is not typically the first affliction suspected of being the cause of many people's illness.
Initially, a series of blood tests are done to determine general blood cell concentrations within the body, how the kidneys are functioning, liver functionality, electrolyte levels, and thyroid functionality If these tests all still lend to the diagnosis of AA, then bone marrow tests must be performed in order to determine the true functionality of the bone marrow (Medic, 1997). Bone marrow biopsies are a last performed only after all the other tests have been performed because they are not particularly easy proceedures.
Once a bone marrow biopsy has been performed however, medical professionals check the level of functioning bone marrow to determine if AA is in fact the cause of the disease that is troubling the patient. If there is very little functioning marrow, or a high concentration of fatty marrow tissue, then AA is generally suspected of being the cause (Young 2002).
Treatment
There are two main forms of treatment for AA: immunosupressive therapy and bone marrow transplantation. The most common forms of immunosupressive therapy that are used to treat AA are antilymphocyte globulin, cyclosporine, or both (Tichelli A, et al 1999). If the disease is caught early enough, before the majority of the bone marrow has been depleated, then immunosupressive therapies may be the best option for treatement.
The idea behind the immunosupressive therapy is fairly simple: stop the immune system from functioning fully in order to prevent the further breakdown of hematopoietic bone marrow cells by the effector T-cells that are destroying them. Both antilymphocyte globulin and cyclosporine are highly effective methods for hinding the immune system. There are drawbacks to immunosupressive therapies, however. The biggest of which is that suppressing the immune system can leave the patient highly vulnerable to infectious agents, such as viruses and bacteria. When being treated for AA by means of immunosupressive therapy, patients are typically put on high doses of antibiotics and may be isolated in order to prevent infections from occuring (Leukemia Research Foundation, 2005).
Bone marrow transplantation is a very good treatment for AA, in that more than half the time it cures the disease, but it can be extremely difficult to locate a suitable donor (Medic, 1997).
References
Issaragrisil S, Sriratanasatavorn C, Piankijagum A, Vannasaeng S, Porapakkham Y, Leaverton PE, Kaufman DW, Anderson TE, Shapiro S, Young NS. Incidence of aplastic anemia in Bangkok. The Aplastic Anemia Study Group. Blood 1991, 77 (10) 2166-2168.
Kagan WA, Ascensao JA, Pahwa RN, Hansen JA, Goldstein G, Valera EB, Incefy GS, Moore MAS, Good RA. Aplastic Anemia: Presence in Human Bone Marrow of Cells that Supress Myelopoisis. Proceedings of the National Academy of Sciences of the United States 1976, 73 (8) 2890-2894.
Leukemia Resarch Foundation. 2005. Aplastic Anemia. <http://www.lrf.org.uk/en/1/infdispatapl.html>. Accessed 2006 April 24.
Medic The University of Texas-Houston Medical School. 1997. Aplastic Anemia Answer Book. <http://http://medic.uth.tmc.edu/ptnt/00001038.htm>. Accessed 2006 April 24.
Tichelli A, Socie´ G , Henry-Amar M, Marsh J, Passweg J, Schrezenmeier H, McCann S, Hows J, Ljungman P, Marin P, Raghavachar A, Locasciulli A, Gratwohl A, and Bacigalupo A. Effectiveness of Immunosuppressive Therapy in Older Patients with Aplastic Anemia. Ann Intern Med 1999, 130 (3) 193-201.
Young NS. Acquired Aplastic Anemia. Ann Intern Med 2002, 136 (7) 534-546.
Young NS, Maciejewski J. The Pathophysiology of Acquired Aplastic Anemia. New England Journal of Medicine 1997, 336 (19) 1365-1375.
Zoumbos NC, Gascon P, Djeu JY, Young NS. Interferon is a Mediator of Hematopoietic Supression in Aplastic Anemia in vitro and Possibly in vivo. Proceedings of the National Academy of Sciences of the United States 1985, 82 (1) 188-192.