Overview:
Tapasin is a trans-membrane protein that is located within the endoplasmic reticulum (ER) . Its primary function is to assist in the binding of the MHC I protein with its antigen peptide fragment. It acts as an accessory molecule, binding to both the TAP and the MHC I protein within the lumen of the ER. More specifically, it binds to the TAP1 molecule of the TAP complex, and the b2m regioin of the MHC I protein. Initially, the alpha chains of the MHC I protein are held into place within the ER by the protein calnexin.
The b2m region then binds to the alpha chains. It is not known whether or not tapasin plays a role in assisting in the binding of the b2m region to the alpha chains, but once the b2m region becomes associated with the MHC I protein, the MHC complex disassociates from calnexin and binds its b2m region with tapasin, which is also bound to TAP1.
Once this binding occurs, chaperone molecules, Erp57 and calreticulin, that assist in the MHC I/peptide formation bind to the alpha chains of the MHC I molecule. As soon as this complex of TAP, tapasin, MHC I, Erp57, and calreticulin is formed, the TAP protein channel opens and allows peptide fragments that have been cleaved by a proteasome external to the ER to enter the ER (see figure 1).
Once the peptide fragments enter the ER, tapasin then assists in the binding of the peptide fragments to the MHC I complex. The MHC I complex is then complete and ready to be exported to the outer cell membrane so that it can present its peptide fragment to immunoglobins (Grandea A G et. al. 2001).
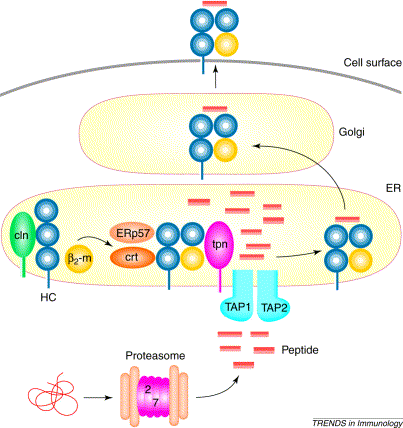
Figure 1: The process of MHC I/peptide binding which takes place within the ER. Foreign proteins are dissolved into peptide fragments by proteasomes outside the ER, and the binding of tapasin with TAP and the MHC I molecule allows the TAP channel to open, which lets the peptide fragments enter the ER. Once inside the ER, tapasin aids in binding the peptide fragments to the MHC I complex. Original image provided by Grandea A G, et. al. (permissioin pending).
Function:
The complete function of tapasin is still yet considered to be unknown, but it has been determined to be associated with three seperate processes so far.
First, and arguably most obvious, is that it allows the MHC I protein and the TAP complex to link to one another within the ER. After the bm2 region of the MHC I protein becomes associated with the alpha sheets that are attached to calnexin, the MHC I molecule dissociates from calnexin, and binds to tapasin. It is thought that tapasin binds to the bm2 portion of the MHC I molecule. Tapasin is believed to be pre-associated with the TAP1 portion of the TAP complex; and thus, when the bm2 portion of the MHC I molecule binds to tapasin, it is linked directly to TAP via tapasin. Without tapasin, there is still some evidence of weak binding between TAP and MHC I, but it is no where near as prominent as it is in cells expresssing tapasin at normal levels (Ortman et. al. 1997).
Second, it is thought that upon binding with tapasin, the MHC I molecule undergoes conformational changes which allow it to better bind to its peptide. (Li et. al. 2000).
And finally, upon binding to the MHC I molecule, it is believed that tapasin causes the upregulation of the TAP1 portion of the TAP complex. Upregulating the TAP1 protein causes the TAP complex itself to become activated, and thus allows peptides to enter the ER. After the peptides enter the ER they are able to bind to the MHC I molecule, which is still bound to tapasin via its b2m region (Ortman et. al. 1997).
After the peptide binds to the MHC I molecule, tapasin releases it, and the other accessory proteins associated with MHC I also move away. The MHC I molecule then exocytoses from the ER, and fuses with the outer cell membrane so that it may be exposed to the extra cellular space, and the peptide fragment from the pathogen infecting the cell can be presented to immunoglobins (Grandea A G et. al. 2001).
Structure:
The structure of tapasin is still somewhat ambiguous, but it is known that it is a transmembrane protein that is 48 kDa in size, and its active portion is located within the interior of the membrane of the ER.
It has been shown to have the ability to bind to TAP in the absence of MHC I. It has also been shown to be able to bind to MHC I in the absence of TAP, thus it is believed that it has two seperate binding sites for each of the two proteins (Sadasivan et. al. 1996).
The N terminus of the tapasin molecule is the region has been shown to be the end that binds to the MHC I protein within the ER. It is believed that it is the 50 last residues of this region that allow for the stable formation of the MHC I, tapasin, TAP complex. It has also been shown that the C terminus of tapasin is the region that binds to the TAP1 molecule (Bangia et. al. 1999).
Problems Related to Tapasin Deficiency:
In the absence of tapasin, it is thought that peptides do not bind properly to the MHC I complex within the ER, and subsequently, expression of MHC I/peptide complexes on the outer membrane of the cell is greatly diminished. As mentioned above, there are weak interactions between the MHC I molecule and the TAP molecule, thus even in the complete absence of tapasin, there is still some MHC I presentation on the external membrane of the cell.
Although there is still some MHC I/peptide presentation in tapasin deficient individuals, their immune resopnse is still affected in a large way by their tapasin deficiency. Their body's adaptive immune defenses against viruses are severly impared, since the peptide fragments are not able to be presented on the outside of the cell as to be recognized by immunoglobins which would target the infected cell (Li et. al. 2000).
So basically, people who are affected with tapasin deficiencies have a severly decreased level of MHC I molecules on the outside of their cells. This symptom is very similar to what is experienced by people with a TAP deficiency. Individuals that have a TAP deficiency are diagnosed with a disease called bare lymphocyte syndrome (BLS). Recently, it has been shown that some individuals who express the symptoms of BLS are actually not TAP deficient; however, they have mutated forms of tapasin, which results in a phenotype very similar to that of a TAP deficient individual: very little MHC I presentation on the outside of the cell (Yabe et. al. 2002).
References:
Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur J Immunol 1999, 29 (6) 1858-70.
Chun T, Grandea A G, Lybarger L, Forman J, Van Kaer L, and Wang C. Functional Roles of TAP and Tapasin in the Assembly of
M3-N-Formylated Peptide Complexes. Jour Immunol 2001, 167 1507-1514.Grandea A G, Van Kaer L. Tapasin: an ER chaperone that controls MHC class I assembly with peptide. Tren Immunol. 2001, 22 (4) 194-199.
Li S, Paulsson K M, Chen S, Sjo¨ gren H, and Wang P. Tapasin Is Required for Efficient Peptide Binding to Transporter
Associated with Antigen Processing. Jor Biol Chem 2000, 275 (3) 1581-1586.Ortmann, B, Copeman J, Lehner P J, Sadasivan B, Herberg J A, Grandea A G, Riddell S R, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science 277: 1306-1309, 1997.
Sadasivan B, Lehner P J, Ortmann B, Spies T, and Cresswell P. Roles of calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 1996, 5 103–114.
Yabe T, Kawamura S, Sato M, Kashiwase K, Tanaka H, Ishikawa Y, Asao Y, Oyama J, Tsuruta K, Tokunaga K, Tadokoro K, Juji T. A subject with a novel type I bare lymphocyte syndrome has tapasin deficiency due to deletion of 4 exons by Alu-mediated recombination. Blood 2002, 100 (4) 1496-1498.