
Lyme Disease
Borrelia burgdorferi
General Information
Lyme disease is a bacterial infection caused by the spirochete Borrelia burgdorferi as depicted in Fig. 1. Spirochete is a category of spiral, corkscrew shaped microorganisms. In addition, Lyme disease is a vector borne illness that is most commonly transmitted to humans by the deer tick, Ixodes Dammini (Telford et al., 429). The ticks are inoculated with the bacteria by feeding on an infected animal resistant to the disease phenotype. B. burgdorferi resides in the tick’s gut and mouth apparatus and enters the human host as the tick feeds. The bacteria first establish an infection in the skin around the tick bite and later disperse throughout the body via the host’s blood circulation. A pregnant woman with Lyme disease runs the risk of infecting her child, because the bacteria are able to cross the placenta and enter the fetus (Duray et al., 65). Depending on the promptness of treatment, the disease can go on to affect the skin, joint tissue, cardiovascular function as well as the central and peripheral nervous system (Straubinger et al., 2000). Early diagnosis of the disease is crucial to a successful treatment and often involves both the ELISA and Western blott techniques.
Figure 1: A micrograph of the spirochete bacteria
Borrelia burgdorferi. Note the spiral shape
of each bacterium. This image was borrowed
from the following address with the permission
of theCDC.
<http://www.cdc.gov/ncidod/dvbid/Bburgdorferi.htm>
Aberrant vs. Wild Type Phenotype
There are three progressive stages associated with
Lyme disease each harboring its own array of symptoms. The bite of an infected
tick and the successful transfer of B. burgdorferi to the human are followed
by an inflammatory response, generally one to three weeks later, around
the site of inoculation. This primary erythematous skin lesion is the distinguishing
phenotype of stage I and is called erythema migrans, EM (Duray et al.,
4). Generally reddish in color, the EM lesion may develop a faded center.
Enlarging of the EM, as shown in Fig. 2, is indicative to the duration
of the disease. Primary EM lesion usually heals spontaneously within weeks,
but may persist for up to one year (Asbrink et al., 4). As the bacteria
randomly spread hematogenously, smaller secondary lesions appear throughout
the skin and the disease has now progressed to stage II. Just as the primary
lesions, these secondary ones heal spontaneously. A second symptom of stage
II is Borrelia lymphocytoma, localized hardening of the dermis, prominent
in the ear lobes and the nipples (Duray et al., 65). More threatening traits
of the second stage involve the cardiovascular and central nervous systems.
Cardiac arrhythmia and cranial neuritis are prevalent among Lyme disease
patients (Asbrink et al., 4). The final stage of Lyme disease is associated
with chronic bouts of arthritis in the joints and sensorimotor neuropathies.
Lyme arthritis generally involves the knee, shoulder and wrist and results
from the build up of synovial fluid. This fluid has high concentrations
of lymphocytes, plasma cells, macrophages and mast cells. Though usually
prevalent in the synovial fluid, there are no neutrophils in the synovium
of patients with Lyme arthritis (Duray et al., 65). The sensorimotor neuropathies
of stage III result in nerve fiber loss and axonal degeneration such as
demyelination as shown in Fig. 3. It is interesting to note that patients
in stage III of Lyme disease often complain of memory loss (Duray et al.,
65).
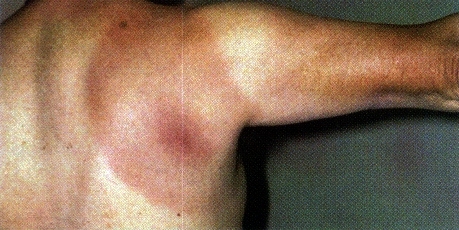 Figure 2: This patient is displaying one of the most prevalent
signs of stage I lyme disease, erythema migran rashes. This image
was borrowed from the following address with the permission of the CDC.
|
 Figure 3: This is a micrograph of axonal loss, specifically active
demyelination. The arrow is pointing to a demyelinated axon surounded by
a phagocytic cell. This image was borrowed from the following address pending
the permission of its author Robert Schmidt MD.
|
Human Immune Response to Borrelia Burgdorferi Infection
Prior to eliciting an immune response, the bacteria
must first establish a site of infection in the skin. After crossing the
dermis, the B. burgdorferi elicits virtually all aspects of the human immune
response. Despite alternative and classical compliment activation, a vigorous
CD4 T cell and humoral response, and NK activity, the bacteria is able
to persist in the patient. As a result, Lyme disease is often associated
with chronic symptoms as the bacteria enter stages of quiescence.
Once transmitted to the skin the compliment system
is among the first barriers B. burgdorferi encounters. As expected the
bacteria activates the alternative compliment pathway, but is also able
to activate the classical compliment pathway before a population of monoclonal
antibodies specific for B. burgdorferi has been established (Suhonen et
al., 2000). Following compliment activation are the three affecter functions
of the compliment system, opsonization of pathogens, lysis of pathogens
and the recruitment of phagocytic cells (Janeway et al., 1999).
Neutrophils are among the first phagocytic cells
to arrive at the site of infection and proceed to activate bactericidal
mechanisms such as oxidative burst, calcium mobilization and phagocytosis.
The oxidative burst is similar to the respiratory burst during which bactericidal
agents such as NO, H2O2, and O2-
are produced and released by the neutrophils and macrophages (Janeway et
al., 1999). Phagocytosed B. burgdorferi are usually bound to C3b, which
is specific to the surface receptor CR3 found on the neutrophils. It has
been shown that both calcium mobilization and the oxidative burst initiated
by neutrophils are absolutely compliment dependent. Though not dependent
on it, the neutrophils’ phagocytic activity against B. burgdorferi is greatly
enhanced by compliment activation (Suhonen et al., 2000).
Virtually without exception the compliment is unable
to rid the body of the B. burgdorferi infection and is followed by a rapid
CD4 T cell and later a humoral response. In an established B. burgdorferi
infection there is a high frequency of T cells, 1 out of 3700, specific
for the whole organism. Test subjects injected with centrifuged samples
of B. burgdorferi showed a lower frequency of T cells specific to the supernatant
matter. These results imply that there are many mitogenic peptides located
on the bacteria’s surface membrane (Dattwyler et al., 93). Some of the
peptides prone to MHC II presentation are also common antigens for the
humoral response.
Both the flagella and outer surface proteins of
B. burgdorferi are involved in cytoadherence and both are common antigens
to different classes of IgG antibodies (Benach et al., 115). The outer
surface proteins, Osps, as seen in Fig. 4 have carbohydrates attached to
them. The subclasses of IgG antibodies involved in the humoral response
against B. burgdorferi are in decreasing prominence IgG1, IgG3>IgG2>IgG4
(Hechemy et al., 162). The populations of both IgG 1 and IgG3 are binding
protein antigens, many of which are associated with the bacteria’s’ flagella.
The IgG 2 antibody populations are significantly smaller and often do not
develop until the late stages of Lyme disease. These IgG 2 antibodies are
binding primarily to the carbohydrate groups associated with the Osps of
B. burgdorferi (Batsford et al., 1998). IgG1 and IgG 3 subclasses predominately
function as markers for opsonization, whereas IgG 2 antibodies neutralize
their antigen (Janeway et al., 1999).
B. burgdorferi coated with antibody could be susceptible
to Natural Killer, NK, cells. Though normally associated with natural resistances
to tumors, viral infections, and transplant rejections, Golightly et al.
have shown that NK cells play a role in host defense against bacterial
infections (Golightly et al., 103). Lyme disease patients not treated with
antibiotics early on in the course of infection have fourfold increases
in their NK cell levels. Such evidence is strong support for NK cells’
role against bacterial infection. However, despite the fourfold increase
in NK cell levels, the NK cell activity is decreased by 30-40% in Lyme
disease patients (Golightly et al., 103). It is currently thought that
actively dividing B. burgdorferi secrete an adenylate cyclase that increases
the cAMP levels, which in turn inhibits the activity of, but does not kill
the NK cells (Golightly et al., 103).
Figure 4: This is the outer surface protein A located on the
bacteria Borrelia burgdorferi that causes Lyme disease.The Osps
are especially important in the cytoadherence of the bacteria to
host cells.
Treatment of Lyme Disease
Early diagnosis is the most important aspect to treating
Lyme disease. The longer the disease progresses the more susceptible the
patient is to chronic symptoms caused by B. burgdorferi that have infected
immunologically privilaged sites. As a bacterial infection the most common
prescribed treatments for Lyme disease are antibiotics. Penicillins, cell
wall inhibitors, and tetracyclines, protein synthesis inhibitors, are two
classes of antibiotics used against Lyme disease. However, B. burgdorferi
can secrete a glycoprotein that encapsulates and shields it from the antibiotics.
Secondly, B. burgdorferi often enters a quiescence stage once it enters
macrophages, neurons and fibroblasts (Golightly et al., 103). Since antibiotics
target active bacteria, only during the growth phase, thought to occur
every four weeks, are these quiescent B. burgdorferi susceptible to the
antibiotics (Burrascano 1998). Consequently, patients with Lyme disease
are prescribed antibiotics for a minimum of four weeks. For those patients
with late stage Lyme disease antibiotics may be prescribed for four to
six months (Burrascano 1998). As with any bacterial infection, not completing
the prescribed antibiotic regiment makes the patient more susceptible to
a relapse of the disease.
In conjunction with antibiotic treatment, patients
may also be vaccinated with recombinant Osp A. Luke et al. have shown that
immunization with Osp A confers some resistance to B. burgdorferi (Luke
et al., 2000). This resistance is derived from the antibodies specific
for Osp A that inhibit the growth of B. burgdorferi. Recall that Osp A
is critical for the cytoadherence of B. burgdorferi to its host cells (Luke
et al., 2000). Vaccines of recombinant Osp A are only a supplement to the
antibiotic treatments due to the multiple epitopes of Osp A. For instance,
many of the antibody populations elicited by the vaccine may be specific
to epitopes on Osp A that are not associated with bacterial growth inhibition
(Luke et al., 2000).
Works Cited:
1. Asbrink, Eva and Anders Hovmark. "Early and Late Cutaneous Manifestations
in Ixodes-borne Borreliosis (Erythema Migrans Borreliosis, Lyme
Borreliosis)." Lyme Disease
and Related Disorders. Annals of the New York Academy of Sciences.
Ed. Jorge L. Benach and Edward M. Bosler.
Vol.539. New York: The New
York Academy of Sciences, 1988: 4-16.
2. Batsford, Stephen et al. "Analysis of Antibody Response to the Outer
Surface Protein Family in Lyme Borreliosis Patients." The Journal of
Infectious Diseases
178 (1998): 1676-1683.
3. Benach, Jorge J et al. "Biological Activity of Borrelia burgdorferi
Antigens." Lyme Disease and Related Disorders. Annals of the New
York Academy
of Sciences. Ed. Jorge L.
Benach and Edward M. Bosler. Vol.539. New York: The New York Academy of
Sciences, 1988: 115-125.
4. Burrascano, Joseph J. "The New Lyme Disease: Diagnostic Hints and
Treatment Guidelines for Tick Borne Illnesses." 1998.
<http://www.LymeNet.org/>
Accessed 2000 April 20.
5. Dattwyler, Raymond J. "Specific Immune Responses in Lyme Borreliosis:
Characterization of T Cell and B Cell Responses to Borrelia burgdorferi."
Lyme Disease and Related
Disorders. Annals of the New York Academy of Sciences. Ed. Jorge L.
Benach and Edward M. Bosler.
Vol.539. New York: The New
York Academy of Sciences, 1988: 93-102.
6. Duray, Paul H, and Allen C. Steere. "Clinical Pathological Correlations
of Lyme Disease by Stage." Lyme Disease and Related Disorders. Annals
of the
New York Academy of Sciences.
Ed. Jorge L. Benach and Edward M. Bosler. Vol.539. New York: The New York
Academy of Sciences, 1988:
65-79.
7. Golightly, Marc et al. "Modulation of Natural Killer Cell Activity
by Borrelia burgdorferi." Lyme Disease and Related Disorders.
Annals of the New York
Academy of Sciences. Ed.
Jorge L. Benach and Edward M. Bosler. Vol.539. New York: The New York Academy
of Sciences, 1988: 103-111.
8. Hechemy, Karim E et al. "Immunoglobulin G Subclasses Specific to
Borrelia
burgdorferi in Patients with Lyme Disease." Lyme Disease and Related
Disorders. Annals
of the New York Academy of Sciences. Ed. Jorge L. Benach and Edward M.
Bosler. Vol.539. New York: The New York
Academy of Sciences, 1988:
162-169.
9. Janeway CA, Travers PT, Walport M, Capra JD.1999. Immunobiology:
The immune system in health and disease 4th ed. Union Square
West, New
York, NY: Elsevier Science
Ltd/Garland Publishing. p 326, 335, 341.
10. Luke, Catherine et al. "Growth-Inhibiting Antibody Response of Humans
Vaccinated with Recombinant Outer Surface Protein A or Infected with
Borrelia burgdorferi
or Both." The Journal of Infectious Diseases 181 (2000): 1062-1068.
11. Lyme Disease: Introduction. CDC: Center for Disease Control and
Prevention. 1999 June 4. <http://www.cdc.gov/ncidod/dvbid/lymeinfo.htm>
Accessed 2000 April 17.
12. Schmidt, Robert. Peripheral Nerve: Chronic Demyelination. 1999 May
21. <http://www.neuro.wustl.edu/neuromuscular/pathol/nervedem.htm>
Accessed 2000 April 17.
13. Straubinger, Reinhard K et al. "Status of Borrelia burgdorferi
Infection after Antibiotic Treatment and the Effects of Corticosteroids:
An
Experimentaln Study." The
Journal of Infectious Diseases 181 (2000): 1069-1081.
14. Suhonen, Juha et al. "Borrelia burgdorferi-Induced Oxidative
Burst, Calcium Mobilization, and Phagocytosis of Human Neutrophils are
Complement Dependent." The
Journal of Infectious Diseases 181 (2000): 195-202.
15. Telford, Sam R et al. "Incompetence of Deer as Reservoirs of Borrelia
burgdorferi." Lyme Disease and Related Disorders. Annals of
the
New York Academy of Sciences.
Ed. Jorge L. Benach and Edward M. Bosler. Vol.539. New York: The New York
Academy of Sciences,
1988: 429-430.