Cell, Vol. 69, 237-249, April 17, 1992, Copyright © 1992 by Cell Press
*Howard Hughes Medical Institute
Department of Genetics and Development
Columbia University College of Physicians and Surgeons
New York, New York 10032
+ MRC Laboratory of Molecular Biology
Hills Road
Cambridge CB2 2QH
England
Summary
Most of the thorasic and abdominal segments of Drosophila are specified early in embryogenesis by the overlapping activities of the hunchback (hb ), Krüppel, knirps, and giant gap genes. The orderly expression of these genes depends on two maternal determinants: bicoid, which activates hb transcription anteriorly, and nanos, which blocks translation of hb transcripts posteriorly. Here we provide evidence that the resulting gradient of hb protein dictates where the Krüppel, knirps, and giant genes are expressed by providing a series of concentration thresholds that regulate each gene independently. Thus, hb protein functions as a classical morphogen, triggering several distinct responses as a function of its graded distribution.
Introduction
One of the central problems in developmental biology is to explain how the body plan is first established. In Drosophila, most of the components required for specifying the basic pattern of head, thorasic, and abdominal segments have been identified, and in many cases their roles and modes of action have been determined (reviewed in Nüsslein-Volhard, 1991). Yet major uncertainties remain. One is the global control of thorasic and abdominal segmentation.
Proper development of the posterior half of the body normally depends on the activity of the maternal determinant nanos (nos ) (Nüsslein-Volhard et al.,1987; Lehmann and Nüsslein-Volhard, 1991). nos mRNA is tightly localized at the posterior pole of the fertilized egg and is presumed to give rise to a gradient of nos protein soon after fertilization (Wang and Lehmann, 1991). Moreover, both genetical and embryological studies suggest that nos can function as a graded morphogen that specifies abdominal pattern (Lehmann and Nüsslein-Volhard, 1986; Wharton and Struhl,1991). However, under certain conditions, the normal pattern of abdominal segments can be formed in the absence of nos activity (see below); hence, some other factor must be capable of generating posterior body pattern independently.
The best candidate for this factor is hunchback (hb ) protein. The hb gene is transcribed during oogenesis, and the resulting transcripts are distributed uniformly in the egg (Tautz et al., 1987). Soon after fertilization, these transcripts are preferentially translated in the anterior half of the body because nos represses their translation posteriorly (Tautz, 1988). The hb gene is also transcribed under the control of the anterior determinant bicoid (bcd ) (Tautz, 1988; Schröder et al., 1988; Driever and Nüsslein-Volhard, 1989; Struhl et al., 1989). Hence, bcd activates hb anteriorly, whereas nos represses it posteriorly; together, both activities ensure that the concentration of hb protein is maximal in the anterior half of the body and declines to undetectable levels in the posterior half.
The progressive decline in hb protein concentration from high to undetectable levels across the middle portion of the body appears to be critical for generating posterior body pattern. When hb protein is allowed to accumulate inappropriately in the posterior half of the body (e.g., by inactivating nos, deleting cis-acting nos response elements in hb mRNA, or generating high levels of hb transcripts under hsp70 control), abdominal segmentation is blocked (Tautz, 1988; Hülskamp et al., 1989; Struhl, 1989a; Wharton and Struhl, 1991). Conversely, when hb protein expression is prevented in both halves of the body (e.g., by inactivating both the maternal and zygotic hb mRNAs by mutation or by causing ectopic nos activity at the anterior pole), many abdominal segments fail to form or have reversed polarity (Lehmann and Nüsslein-Volhard, 1987; Wharton and Struhl, 1989; Hülskamp et al., 1990). Finally, differential hb expression can suffice to generate posterior body pattern, even in the absence of the posterior determinant nos (Hülskamp et al.,1989; Irish et al., 1989; Struhl, 1989a). In this unusual circumstance, in which maternal hb transcripts are inactivated by mutation thereby obviating a requirement for nos, normal patterning depends on the formation of an hb gradient under bcd control (Hülskamp et al., 1990).
How does differential hb expression generate posterior pattern? The key to answering this question must lie in deployment of the gap genes Krüppel (Kr ), knirps (kni ), and giant (gt ), which are activated in overlapping posterior domains and which control distinct portions of the abdominal segment pattern (Nüsslein-Volhard and Wieschaus, 1980; Carroll and Scott, 1986; Ingham et al., 1986; Frasch and Levine, 1987; Struhl, 1989b). Recent studies of these genes have led to the hypothesis that the anteroposterior differential of hb protein expression constitutes a morphogen gradient specifying where Kr, kni, and gt are expressed (Hülskamp et al., 1990; Eldon and Pirrotta, 1991; Kraut and Levine, 1991a, 1991b; see also Gaul and Jäckle, 1987, 1989). In these studies, the pattern of hb protein expression has been altered by mutations in hb, nos, or bcd ; the resulting changes in the patterns of Kr, kni, and gt expression have suggested that high concentrations of hb protein block expression of all three of these genes, whereas lower concentrations allow Kr activity but still prevent kni and gt expression.
In the experiments described here, we test this hypothesis by creating embryos in which the profile of the hb protein gradient is systematically altered, while all other known signaling systems are eliminated or held constant. In this way, it has been possible to assess how the boundaries of Kr, kni, and gt expression are influenced by the distribution of hb protein. We show that the hb gradient provides a series of distinct concentration thresholds that position the anterior boundaries of expression of all three genes, as well as the posterior boundary of Kr expression. Moreover, we show that these responses are independent and sufficient to generate the overlapping domains of gap gene expression that thereafter specify most aspects of posterior body pattern. Thus, hb controls thorasic and abdominal segmentation by acting as a classical gradient morphogen (Dalcq, 1938; Turing, 1952; von Ubisch, 1953; Sander, 1959, 1960, 1975).
Results
hb Protein Gradient
The early expression of hb protein depends on two independently controlled
sources: maternal mRNAs that are initially distributed throughout the egg
but are not translated posteriorly owing to nos ; and zygotic mRNAs that
are transcribed anteriorly in response to bcd . Together, these sources
generate a pattern of differential hb expression in which the concentration
of protein peaks in the anterior half of the body under bcd control and
declines in a graded fashion to undetectable levels in the posterior half
under nos control (Figure 1). Because nos and bcd act in distinct domains
and have opposite effects on hb expression, the hb gradient can be
subdivided into a lower (nos-dependent) and upper (bcd-dependent) half,
each of which can be analyzed independently.

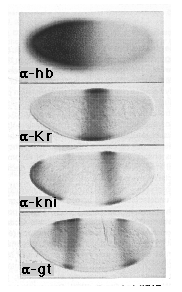
Figure 1. hb, Kr, kni, and gt Protein Expression in Wild-Type
Embryos
The pattern of hb protein expression is shown in an embryo prior to nuclear
cycle 13: the plane of focus is at the surface to illustrate the graded
distribution of hb protein in the middle portion of the body. Note that
the concentration of hb protein falls from maximal to undetectable levels
over a distance of approximately 15 nuclei (the distance along the anteroposterior
axis is around 80 nuclei in this embryo); detectable expression extends
approximately two thirds of the way down the body. The patterns of Kr, kni,
and gt protein expression are shown in mid-cycle 14 embryos (stage 5 (2);
Lawrence and Johnston, 1989; the embryos are shown in optical cross section).
Note that kni and gt are expressed in anterior as well as posterior domains.
For both genes, anterior expression is completely dependent on transcriptional
activation by bcd and falls within the domain of maximal hb expression (also
under bcd control). In contrast, posterior kni and gt expression, as well
as central Kr expression, depends critically on the progressive decline
of hb protein beneath distinct threshold concentrations, which in turn depends
on the absence of bcd activity. Here, and in the remaining figures, all
embryos are oriented with the anterior end at the left and the dorsal side
at the top.
As shown in Figure 1, the hb gradient extends across a central interval of the body in which Kr, kni, and gt are expressed in a series of overlapping domains. In the experiments described here we are concerned principally with the role of the hb gradient in positioning the boundaries of central Kr and posterior kni and gt expression that falls within this interval. The Kr, kni, and gt genes are also activated anteriorly by bcd and repressed at the ends of the body by the "terminal" determinant system (see Nüsslein-Volhard et al., 1987; Nüsslein-Volhard, 1991). As described below, these additional levels of control have been eliminated by appropriate mutations in bcd and either torso (tor ) or torso-like (tsl ), two genes equally essential for terminal specification.
Control by the nos-Dependent Portion of the hb Gradient
In embryos lacking both bcd and tsl (or tor ) function,
hb protein derives solely from maternal hb transcripts that are uniformly
distributed throughout the egg. Hence, the hb protein distribution can be
manipulated simply by varying the maternal hb gene dosage or by eliminating
nos activity, the only remaining regulator of hb expression. As shown in
Figure 2 (left column), we performed three experiments to alter systematically
the distribution of hb protein in these embryos. First, we eliminated hb
protein expression by generating females with hb mutant germ cells
(0x ). Second, we varied the number of maternal copies of the hb gene
from 1 to 2 to 4 (1x, 2x, 4x ), thereby generating a series of gradients
with different profiles (Figure 2 and Experimental Procedures). Third, we
used a mutation in the oskar (osk ) gene to eliminate nos
activity (Lehmann and Nüsslein-Volhard, 1991), thereby causing hb protein
to be expressed at uniform levels throughout the body. As described below,
the distribution of Kr, kni, and gt protein in these embryos, as well as
the resulting segmentation patterns, indicates that the nos-dependent portion
of the hb gradient specifies abdominal segmentation by positioning the posterior
Kr, anterior kni, and anterior gt boundaries.
Figure 2. Control of the Posterior Kr and Anterior gt Boundaries
by the hb Gradient
The patterns of hb, Kr, and gt protein expression are shown in embryos derived
from bcd tsl females carrying 1, 2, or 4 copies of the hb gene
(1x, 2x, and 4x) or containing 2 copies of the hb gene but lacking
nos activity, owing to mutation in the gene osk (osk-)
Also shown in the top row are embryos derived from tor ; bcd hb
oocytes obtained by pole cell transplantation (0x). As described in
the text, these embryos are equivalent to embryos derived from bcd tsl
females in that they lack both bcd and tor function; they
differ, however, by the complete absence of hb protein. Note that the level
of hb protein expression correlates with the maternal gene dosage in 1x,
2x, and 4x embryos (e.g., compare the intensity of hb staining in the anterior
half of each embryo). The posterior limit of detectable hb protein expression
also shifts posteriorly with each increase in gene dosage. (Because of the
difficulties in recording low levels of protein expression photographically,
we have measured the posterior limit of detectable hb protein expression
directly; it falls at 39%, 35%, and 28% egg length [measured from the posterior
pole] in 1x, 2x, and 4x embryos, respectively; see Experimental Procedures.)
Note also that Kr and gt expression depend critically on the presence or
absence, respectively, of hb protein and that the boundaries of each are
positioned progressively more posteriorly as the maternal hb copy
number rises from 1 to 2 to 4 (falling at 58%, 50%, and 45% egg length for
Kr, and at 60%, 53%, and 47% egg length for gt ; see Experimental Procedures).
Finally, note that the posterior boundary of detectable hb protein expression
determined by direct measurement extends at least 15% egg length further
posteriorly than the Kr and gt boundaries measured in sibling embryos. All
of the embryos showing Kr and gt protein expression are at the same stage
of nuclear cycle 14 (stage 5(2)); however, all of the embryos showing hb
expression are at an earlier stage (nuclear cycle 11 or 12).
Posterior Kr Boundary
As shown in the middle column of Figure 2, we find that the distribution
of Kr protein depends on that of hb protein. In the absence of hb protein
(0x), Kr is not expressed. In contrast, when a single hb gene is
present maternally, Kr is expressed in a broad anterior domain, extending
about halfway down the body (1x ). Moreover, each increase in hb gene
dosage (2x, 4x) causes a posterior shift in the boundary of Kr expression.
Finally, ubiquitous hb expression (osk-) gives rise to ubiquitous
Kr expression. These results show that in the absence of the anterior and
terminal determinants, a minimum concentration of hb protein is both necessary
and sufficient for Kr gene activity. They also show that the graded
distribution of hb protein controls where the boundary of Kr gene
expression is positioned, presumably by determining where this concentration
threshold occurs.
Anterior gt Boundary
The hb gradient also appears to position the anterior gt boundary. As shown
in the right column of Figure 2, gt is expressed ubiquitously in the absence
of hb protein (0x). However, when a single hb gene is present maternally,
gt expression is blocked in a broad anterior domain (1x). Further, each
increase in maternal hb gene dosage (2x, 4x) is accompanied by a
posterior shift in the boundary of gt expression. Finally, ubiquitous hb
expression (osk-) prevents any detectable gt expression. Thus, in
the absence of bcd and tor activity, gt is only expressed where the concentration
of hb protein falls beneath a critical threshold. As in the case of Kr,
this threshold dependence provides the means by which the hb gradient controls
the pattern of gt expression.
Independent Control of the Posterior Kr and Anterior gt Boundaries
As shown in Figure 2, the Kr and gt genes are expressed in
reciprocal anterior and posterior domains under hb control. Hence, hb might
govern one of these genes directly, which in turn could control the other.
Alternatively, it might deploy each gene independently, activating one (Kr
), while repressing the other (gt ). To distinguish between these
possibilities, we have asked whether hb can control the expression of each
gene in the absence of the other.
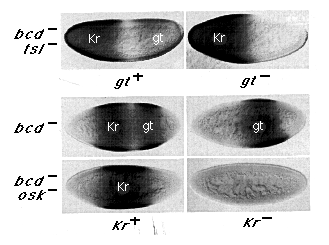
Figure 3. The Posterior Kr and Anterior gt Boundaries Are
Positioned Independently by the hb Gradient
All six embryos shown were double stained for both Kr and gt protein expression
using different immunohistochemical detection systems to generate distinguishable
brown or blue-gray signals: the domains of Kr and gt protein expression
are labeled on the micrographs. All of the embryos shown are at the same
stage of nuclear cycle 14 (stage 5(2)). The upper panel shows that the posterior
Kr boundary is correctly defined in bcd tsl embryos, irrespective
of gt gene activity. The lower panel shows that the anterior gt
boundary is initially set by the hb gradient, irrespective of Kr
gene activity. In this case, gt expression has been compared in embryos
derived from bcd or bcd osk females, which differ principally
in the distribution of hb protein (hb protein expression is graded in bcd
embryos [as it is in bcd tsl embryos, Figure 2] and uniform in
bcd osk embryos [as it is in bcd osk tsl embryos in Figure
2]). Note that gt protein is expressed in a posterior stripe in bcd
embryos but is not detected in bcd osk embryos, whether they
are Kr- or Kr +. This:shows that the ability of the hb gradient
to define initially the anterior gt boundary is not dependent on Kr gene
activity. Note, however, that the anterior gt boundary has begun to shift
anteriorly in the Kr- embryo (as compared with its Kr+ sibling).
As described in the text, the boundary shifts progressively during the latter
portion of nuclear cycle 14, indicating that Kr gene activity plays
a significant role in maintaining the boundary initially defined under hb
control.
We tested the ability of hb to define the posterior Kr boundary in the absence of gt by generating gt- embryos from bcd tsl mutant females. As shown in Figure 3 (upper panel), the absence of gt activity has little effect on the posterior boundary of Kr expression, indicating that the hb gradient positions this boundary independently.
Then we tested the ability of hb to define the anterior gt boundary in the absence of Kr by comparing gt expression in Kr- embryos derived from bcd or bcd osk females (lower panel, right half). In both classes of embryos, early hb protein expression derives exclusively from maternal transcripts. However, in bcd embryos, expression of hb protein is down-regulated posteriorly under nos control (e.g., as in 2x embryos in Figure 2), whereas it persists throughout bcd osk embryos, owing to the absence of nos activity (e.g., as in osk embryos in Figure 2). As shown in Figure 3, the down-regulation of hb in bcd embryos is associated with posterior gt expression, whereas gt is completely repressed in bcd osk embryos where this down-regulation does not occur. More importantly, the same results are observed whether the embryos are Kr+ or Kr-. Hence, hb can repress gt expression and thereby define the anterior gt boundary irrespective of Kr gene activity.
This result does not eliminate the possibility that Kr gene activity may reinforce and stabilize the gt boundary in later embryos. Indeed, we observe a late change in gt expression in Kr embryos derived from bcd females that does not occur in their Kr+ siblings: during the latter portion of nuclear cycle 14 (stages 5(2) and 5(3); Lawrence and Johnston, 1989), the anterior gt boundary shifts progressively anteriorly (e.g., compare gt expression in Kr+ and Kr- embryos derived from bcd females in Figure 3 in which a small shift is already apparent). Thus, although the anterior boundary of gt expression is initially positioned by the hb gradient, Kr gene activity is nevertheless required at a later time to maintain the position of the boundary.
Anterior kni Boundary
The posterior of the two kni domains normally falls between the central
Kr and posterior gt domains, overlapping both but having distinct anterior
and posterior boundaries (see Figure 1). We have tested the possibility
that the hb gradient provides a distinct threshold that independently positions
the anterior kni boundary by examining kni expression in embryos derived
from bcd tsl females carrying 1, 2, or 4 copies of the hb gene.
In the anterior third of these embryos, Kr is "on," whereas
gt is "off," irrespective of whether they derive from females
carrying 1, 2, or 4 copies of the hb gene (see Figure 2). In contrast,
as shown in Figure 4, embryos derived from bcd tsl females with a
single hb gene copy (1x) express readily detectable levels of kni
protein anteriorly. However, barely detectable levels are observed in embryos
from 2-copy females (2x), and no anterior expression is found in embryos
from 4-copy females (4x). Thus, the hb gradient can provide a concentration
threshold that independently positions the anterior kni boundary. This threshold
is clearly distinct from the thresholds governing the neighboring Kr and
gt boundaries.
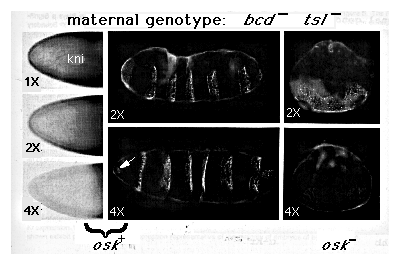
FIG 4. Control of kni Expression and Thorasic versus Abdominal
Differentiation by hb
The panel on the left shows kni protein expression at the anterior end of
embryos derived from bcd tsl females carrying 1, 2, or 4 copies of
the hb (1x, 2x, and 4x). Note that the level of kni protein defines
progressively to undetectable levels as the maternal hb gene dosage
is increased from 1 to 4 copies. High levels of kni protein can be seen
accumulating in the middle portions of these embryos owing to the decline
in maternally derived hb protein. As expected, the position of the interface
between low and high kni expression, like that between anterior Kr and posterior
gt expression (Figure 2), shifts posteriorly in response to increasing the
maternal gene dosage. The middle panels show the segmentation patterns of
pharate first instar larvae derived from bcd tsl females carrying
2 or 4 copies of hb (2 x, 4 x ). Note that both larvae form a polarized
series of several segments. In the 2 x larva, all of these segments form
bands of thick ventral hairs typical of normal abdominal segments; however,
the anteriormost segment in the 4 x larva (arrow) has formed a cluster of
fine hairs characteristic of a thorasic segment. The panels on the right
show larvae derived from embryos in which nos activity is also absent (owing
to mutation in osk ); note that these larvae have formed a lawn of
unpolarized hairs lacking any overt sign of segmentation. Note also that
the hairs are of the abdominal type when the mother carried 2 hb copies
and of the thorasic type when the mother carried 4 copies.
Abdominal Segmentation
As shown above, the low end of the hb gradient is both necessary and sufficient
to specify the orderly expression of Kr, kni, and gt in a series of overlapping
domains. When hb protein is either not expressed or ubiquitously expressed,
these genes respond homogeneously, either by b,being turned on or off throughout
the body. Differential hb repression appears to be equally critical for
abdominal patterning. Embryos derived from bcd tsl females carrying
copies of the hb gene make a polarized series of up to seven abdominal
segments resembling the first seven abdominal segments of the wild-type
larva (2x, Figure 4; see also Nüsslein-Volhard, 1991). However, when
hb protein derived from two gene copies is expressed ubiquitously in embryos
lacking bcd and tor function (e.g., owing to a block in nos activity caused
by the osk mutation; 2x, Figure 4; see also Nüsslein-Volhard,
1991), or not at all (e.g., in embryos obtained from tor ; bcd
hb nos oocytes; data not shown; see Experimental Procedures), they give
rise to an unpolarized lawn of abdominal hairs. Thus, it is the differential
expression of hb protein that specifies abdominal pattern, presumably by
its ability to generate spatially restricted patterns of Kr, kni,
and gt gene expression.
Control by the bcd-Dependent Portion of the hb Gradient
As shown above, the nos-dependent portion of the hb gradient provides distinct
thresholds that set the anterior gt and posterior Kr boundaries of expression.
Moreover, the anterior boundary of kni appears to be dictated by a threshold
concentration that is close to the maximal protein concentration normally
derived from maternal hb transcripts. In the anterior half of the
body, the concentration of hb protein increases far above this concentration,
owing to zygotic activation of the hb gene under bed control. In
the experiments described below, we examined the role of the bcd-dependent
portion of the hb gradient in specifying thorasic as opposed to abdominal
differentiation and in positioning the anterior boundary of Kr expression.
Thorasic Differentiation
As described above, embryos derived from bcd tsl females develop
a polarized series of up to seven abdominal segments resembling the first
seven abdominal segments of the wild-type larvae. As shown in Figure 2,
the concentration of hb protein expressed anteriorly in these embryos can
be increased by doubling the maternal gene dosage from 2 to 4. Under these
conditions, we find that the anteriormost segment usually develops as a
thorasic rather than an abdominal segment (e.g., as seen in the 4x osk+
embryo in Figure 4), suggesting that a 2-fold increase in the concentration
of hb protein is sufficient to dictate a switch from abdominal to thorasic
differentiation. We have tested this possibility by examining embryos derived
from bcd osk tsl females carrying 2 or 4 copies of hb . As
shown in the right column of Figure 4, embryos derived from 2-copy females
(2 x ) differentiate a lawn of unpolarized abdominal hairs, while those
derived from females carrying 4 copies (4x) differentiate a lawn of thorasic
hairs. This 2-fold increase in the concentration of hb protein therefore
appears to specify thorasic as opposed to abdominal differentiation. In
wild-type embryos, this higher level of hb protein expression would require
zygotic activation of the hb gene by bcd, indicating that the bcd-dependent
portion of the hb gradient is responsible for specifying thorasic as opposed
to abdominal differentiation. It is notable that the concentration threshold
that dictates the choice between abdominal and thorasic differentiation
coincides approximately with that required for completely repressing the
kni gene (Figure 4).
Anterior Kr Boundary
To study the role of the bcd-dependent portion of the hb gradient in positioning
the anterior Kr boundary, we have analyzed Kr expression in embryos lacking
nos and tor activity. In these embryos, hb protein is expressed at high
levels anteriorly (under bcd control) and at moderate levels posteriorly
(owing to the absence of nos ). As shown in Figure 5, Kr protein accumulates
in these embryos in a reciprocal pattern, off anteriorly and on posteriorly.
Moreover, the overlapping and opposite distributions of hb and Kr protein
expression observed at high magnification (Figure 5, top panel) are consistent
with the notion that the distribution of hb protein defines the anterior
Kr boundary. We therefore asked whether the position of this boundary depends
on zygotic hb activity. As shown in Figure 5, the anterior Kr boundary shifts
anteriorly in hb mutant embryos obtained from tor; nos
females, establishing such a role.
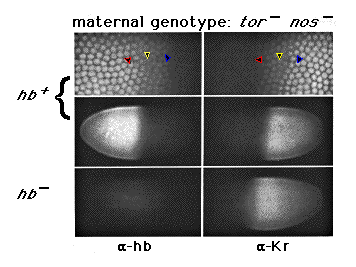
Figure 5. Zygotic hb Activity Plays a Significant Role
in Defining the Anterior Kr Boundary
Sibling hb + and hb - embryos derived from tor ; hb
nos /nos mutant females are shown, double labeled, for hb
and Kr protein expression. Both embryos are at the same stage
of nuclear cycle (stage 5(2)); at this stage, we can no longer detect hb
protein expression derived from maternal hb transcripts by immunofluorescence.
At higher magnification (top panel), the patterns of zygotically derived
hb and Kr protein expression appear reciprocal (colored arrowheads
mark the same nuclei in each micrograph). Note that the Kr boundary
shifts anteriorly in the embryo lacking zygotic hb activity, but
does not extend all the way to the anterior pole. The remaining restriction
in Kr expression must be due to repression by other factors under
bcd control, since it is not observed in the absence of bcd function
(e.g., as in embryos derived from bcd osk tsl females, Figure 2).
One simple hypothesis to account for the control of the anterior Kr boundary is that high levels of hb activity could block Kr expression, just as lower levels suffice to block kni and gt expression. We have tested this as follows. Embryos were obtained from females that are triply mutant for three genes: vasa (vas ), tor, and exuperantia (exu ). The vas mutation blocks nos activity and, hence, is equivalent to a mutation in nos itself (Lehmann and Nüsslein-Volhard, 1991). The mutation in the exu gene interferes with the normal localization of bcd transcripts at the anterior pole (Berleth et al., 1988); the delocalized transcripts give rise to a shallow gradient of bcd protein that spans the anteroposterior axis (Frohnhöfer and Nüsslein-Volhard, 1987; Driever and Nüsslein-Volhard, 1988; Struhl et al., 1989). In embryos derived from vas tor exu females, this gradient becomes the sole determinant of anteroposterior pattern, owing to the absence of both nos and tor activity. As shown in Figure 6, hb protein is expressed at uniform and high levels in these embryos, from which we infer that the concentration of bcd protein is sufficiently high throughout the body to activate the hb gene maximally. Under the hypothesis above, Kr expression should be completely repressed in these embryos. However, the majority of these embryos express Kr protein posteriorly (Figure 6). Hence, the simple explanation that peak levels of hb protein suffice to block Kr expression is inadequate.
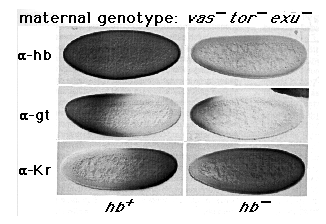
Figure 6. The Position of the Common Border between gt
and Kr Expression in vas tor exu Embryos Depends on the Concentration of
hb Protein
The patterns of hb, gt, and Kr protein expression are shown in hb+
and hb- embryos derived from vas tor exu mutant females. All
of the embryos are at the same stage of nuclear cycle 14 (stage 5 (2)).
As shown in the top panel, the level of hb protein expression derived from
maternal hb transcripts is close to the limit of detection at this
stage, in contrast to the level of hb protein expression derived from zygotic
transcripts, which is high. Note that in both cases, hb protein expression
is uniform along the anteroposterior axis. Note also that the gt and Kr
boundaries both shift anteriorly in hb- embryos relative to hb+
embryos. As described in Experimental Procedures, hb- embryos were
recognized as such by an independent marker. Some variation was observed
in the patterns of both gt and Kr protein expression in these embryos. In
particular, in hb+, low levels of gt expression were sometimes detectable
throughout the posterior half of the body, while, conversely, we sometimes
failed to detect posterior Kr expression. We attribute this variation to
small differences in the distribution of bcd protein, particularly its concentration
posteriorly. The embryos shown exhibit patterns of protein expression representative
of the majority of embryos of each genotype.
We next asked whether the position of the anterior boundary of Kr expression observed in vas tor exu embryos depends on the concentration of hb protein, as is the case for the Kr boundary in tor ; nos embryos (Figure 5). As shown in Figure 6, this is indeed the case: the anterior boundary of Kr expression shifts forward in these embryos in the absence of zygotic hb expression. Thus, it seems that the anterior Kr boundary is positioned in response to the distributions of at least two factors: the concentration of hb protein and at least one other factor that acts differentially in the anterior portion of the body.
A likely candidate for such a factor is gt protein, which is activated anteriorly under bcd control and which can repress Kr gene activity when ectopically expressed under the control of the hsp70 promoter (Eldon and Pirrotta, 1991; Kraut and Levine, 1991a, 1991b). As shown in Figure 6, gt is activated in a broad anterior domain in embryos derived from vas tor exu females, presumably in response to the shallow gradient of bcd protein. Moreover, its pattern of expression appears to be reciprocal to that of Kr in sibling embryos. Finally, we observe that the position of the gt boundary depends on zygotic hb gene activity; when this activity is eliminated, gt expression shifts anteriorly in concert with the anterior shift in Kr expression (Figure 6). Thus, the anterior Kr boundary is not positioned simply by the decline in hb protein concentration from peak to intermediate levels. Instead, it may be set in response to the distribution of gt protein, which in turn depends on the distributions of both bcd and hb protein.
Discussion
The key attribute of a morphogen gradient is that the changing concentration of a single molecular species triggers a series of spatially distinct responses governing cell or body pattern (Daloq, 1938; Turing, 1952; von Ubisch, 1953; Sander, 1959, 1960, 1975). The control of head and thorasic segmentation in Drosophila by the bcd protein provides a clear paradigm for such a gradient system. bcd protein has been shown to bind and activate the transcription of at least one target gene, hb, in a concentration-dependent fashion, thereby providing the means by which the bcd gradient controls where hb is expressed (Driever and Nüsslein-Volhard, 1989; Driever et al., 1989; Struhl et al., 1989). Moreover, the bcd gradient clearly has the instructional capacity to dictate other spatially distinct responses by the same mechanism (Driever et al., 1989; Struhl et al., 1989), and a number of potential target genes involved in head and thorasic differentation have been identified (Cohen and Jurgens, 1990; Dalton et al., 1989; Eldon and Pirrotta, 1991; Finkelstein and Perrimon, 1990; Kraut and Levine, 1991a, 1991b). Here we show that a second morphogen gradient controls many aspects of both thorasic and abdominal segmentation. The morphogen in this case is hb protein that is expressed as a gradient under the joint control of bcd and the posterior determinant nos (Figure 7). Our experiments demonstrate that the hb protein gradient controls posterior body pattern by providing several distinct thresholds that govern the domains of expression of the gap genes Kr, kni, and gt.
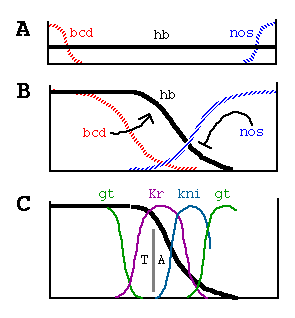
Figure 7. Generation and Function of the hb Morphogen Gradient
(A) bcd and nos maternal transcripts are tightly localized
at the anterior and posterior poles of the unfertilized egg, in contrast
to hb transcripts, which are ubiquitously distributed.
(B) Shortly after fertilization, bcd transcripts are translated,
and the resulting protein diffuses posteriorly, generating a gradient; similarly,
nos transcripts are thought to generate an opposing gradient of nos
activity, presumably its encoded protein. bcd activates zygotic hb transcription
anteriorly, whereas nos translationally represses hb transcripts
posteriorly. As a consequence, hb protein accumulates differentially, declining
in a graded fashion from high, uniform levels at the anterior end to undetectable
levels at the posterior end.
(C) The hb protein gradient then provides a series of concentration thresholds
that independently dictate where the anterior Kr, kni, and gt boundaries,
as well as the posterior Kr boundary, are positioned in the posterior half
of the body. The posterior kni boundary is governed in part by gt (and hence
indirectly by hb ) and by the terminal system, which also controls the posterior
gt boundary. The domain of anterior gt expression (activated under bcd control)
also depends on the concentration of hb protein. Finally, the hb gradient
dictates the boundary between thorasic (T) and abdominal (A) differentiation,
possibly by directly repressing bithorax complex gene activity.
Generation of the hb Gradient by bcd and nos
bcd and nos play distinct, albeit overlapping, roles in generating the hb
gradient. bcd is required for the upper end of the hb gradient, which, we
show here, plays a significant role in positioning the anterior boundaries
of Kr and kni expression and in dictating thorasic as opposed to abdominal
differentiation. Conversely, nos is essential for the lower end, which we
show governs abdominal segmentation by defining the posterior Kr and anterior
kni and gt boundaries. Because both systems influence abdominal segmentation
by their ability to control the concentration of hb protein, it is possible
to create abdominal conditions in which either system can partially or completely
substitute for the other (Huiskamp et al., 1989; Irish et al., 1989; Struhl,
1989a; Figure 4). Nevertheless, in the context of wild-type development,
they each play distinct and essential roles in generating the hb gradient.
hb as a Gradient Morphogen
Soon after the formation of the hb protein gradient, the Kr, kni,
and gt gap genes are activated in a series of overlapping domains,
each having distinct anterior and posterior boundaries located in the region
of the body where hb protein expression declines from maximal to undetectable
levels (e.g., Figures 1, 2, and 7). Prior studies suggested that the graded
distribution of hb protein may influence the activity of these other gap
genes (Hülskamp et al.,1990; Eldon and Pirrotta, 1991; Kraut and Levine,
1991a, 1991b). However, with the exception of the anterior kni boundary
(Hülskamp et al.,1990), these studies failed to provide compelling
evidence for a causal relationship between the changing concentration of
hb protein and the spatial domains of expression of these target genes.
Our experiments establish such a causal relationship for each of the three
genes. Moreover, they show that each gene responds independently to the
hb gradient.
In the case of the bcd gradient, as little as a 2-fold difference in the concentration of bcd protein appears sufficient to distinguish between on or off states of expression of its target genes (Struhl et al.,1989). This inference is based on the observation that successive 2-fold increases in bcd gene dosage shift the posterior boundaries of these target genes by an interval similar to that in which the expression of their products falls from peak to undetectable levels (discussed in detail in Struhl et al., 1989). A comparable relationship is also observed for the hb gradient: as the maternal gene dosage of hb is increased from 1 to 2 to 4, the anterior gt and posterior Kr boundaries shift by intervals of approximately 5%-10% egg length, similar to the interval in which the expression of each declines from maximal to undetectable levels for any given hb gene dosage. Hence, we conclude that relatively small differences in the concentration of hb protein are sufficient to distinguish between "all" or "none" states of subordinate gene expression.
A complicating issue in interpreting the role of the hb gradient is that the gap genes Kr, kni, and gt, once active, engage in extensive cross-regulation that generally tends to reinforce and stabilize the spatial relationships initially established under hb control (Jäckle et al., 1986; Gaul and Jäckle, 1989; Pankratz et al., 1989; Eldon and Pirrotta, 1991; Kraut and Levine, 1991b). For example, the hb gradient can initially define the anterior boundary of gt expression irrespective of Kr gene function (Figure 3). However, this boundary subsequently shifts anteriorly in the absence of Kr activity, reflecting a role for Kr in defining neighb oring boundaries of gt expression (Figure 3; see also Eldon and Pirrotta, 1991; Kraut and Levine, 1991a, 1991 b). This distinction between establishing and stabilizing the orderly expression of gap genes raises the question of when the hb gradient acts. As described previously (Tautz, 1988), the graded distribution of hb protein changes continuously from the first accumulation of protein prior to pole cell formation to the sharply defined bipartite pattern observed just prior to gastrulation. Localized Kr, kni, and gt transcription is first observed during nuclear cycles 11 and 12 (Knipple et al .,1985; Nauber et al., 1988; Eldon and Pirrotta, 1991; Kraut and Levine, 1991a), arguing that graded hb protein is active at this time, if not earlier. Conversely, hb protein derived from maternal transcripts is difficult to detect after the beginning of nuclear cycle 14, suggesting that from this stage on, the nos-dependent portion of the hb gradient is no longer a factor in sustaining the spatial relationships between zygotic hb, Kr, kni, and gt expression.
Cross-regulatory interactions may also allow the hb gradient to define additional boundaries of subordinate gene expression. For example, in embryos lacking both bcd and tor activity, the hb gradient specifies a tripartite pattern of kni expression (low anteriorly, high centrally, and low posteriorly; data not shown). The first boundary, between low anterior and high central expression, is governed by the progressive decline in hb protein concentration. However, the second boundary, between high central and low posterior expression, appears to depend on posterior gt expression, as it is eliminated in the absence of gt gene activity (data not shown). Thus, the hb gradient appears to influence the posterior kni boundary indirectly by defining the gt boundary.
Finally, we note that there is some redundancy in function between the bcd and hb morphogen gradients. As shown previously by Hülskamp et al. (1990), bcd activity can suffice to activate low levels of Kr gene expression in complete absence of hb protein, allowing hb mutant embryos to develop a few middle abdominal segments that they would otherwise not develop. However, the importance of this regulatory interaction is uncertain, because in the complete absence of bcd protein, the hb maternal gradient appears sufficient to activate Kr fully, to dictate the orderly expression of Kr, kni, and gt, and to generate normal abdominal pattern.
hb as Both an Activator and a Repressor of Transcription
A large body of evidence indicates that hb is a transcriptional regulator
that directly binds defined DNA targets and activates or represses gene
expression as a consequence (Tautz et al., 1987; Stanojevic et al., 1989;
Treisman and Desplan, 1989; Small et al., 1991; Hoch et al., 1991; Qian
et al., 1991). It therefore seems reasonable to propose that hb, like bcd,
controls posterior body pattern by acting as a concentration-dependent transcriptional
regulator. However, all of the known and presumed targets of bcd action
appear to be activated in response to bcd protein, whereas in at least two
clear cases, gt and kni, the response to graded hb protein appears to be
transcriptional repression. We therefore suggest that these genes are initially
turned "on" by one or more ubiquitous transcriptional activators
(see also Kraut and Levine, 1991 b) and that the hb protein antagonizes
these activators by interfering either with their binding to the DNA or
with their interactions with other components of the transcriptional machinery.
In contrast, we also show that hb behaves as an activator of Kr transcription. Although this possibility was initially suggested by the experiments of Hülskamp et al. (1990), the directness of the interaction was subsequently called into question by the observation that hb can repress gt, which in turn can repress Kr (Eldon and Pirrotta, 1991; Kraut and Levine, 1991a, 1991b). Although our experiments do not demonstrate that hb directly activates Kr gene expression, they do show that the activation is not mediated indirectly by blocking repression by gt . In the absence of other candidate repressors that might serve such an intermediate role, we suggest that hb functions directly as a transcriptional activator as well as a repressor.
The mode of action of hb in defining the anterior Kr boundary is more complex. Peak levels of hb protein expression are not sufficient to repress Kr expression, although they appear to influence regulatory relationships between bcd, gt, and Kr . As described previously, bcd activates gt, while Kr and gt appear to engage in a relationship of mutual repression (Eldon and Pirrotta, 1991; Kraut and Levine, 1991a, 1991b). Although the mechanism is unknown, the regulatory balance between these factors appears to depend on the concentration of hb protein, providing the means by which the hb gradient positions the boundary between anterior gt and central Kr expression.
Control of Bithorax Complex Gene Expression by the hb Gradient
Regional differentiation of the thorasic and abdominal segments is controlled
in large part by the selective activity of the bithorax complex (Lewis,
1978; Struhl, 1981), the distinction between thorasic and abdominal differentiation
depending primarily on the Ultrabithorax (Ubx ) gene. Hence,
our finding that a 2-fold difference in the concentration of hb protein
is sufficient to cause a discrete switch between abdominal and thorasic
differentiation (Figure 4) raises the possibility that the anterior boundary
of Ubx transcription is controlled directly by the ability of hb
protein to bind and repress transcription of the gene. Indeed, Qian et al.
(1991) identified a cis-acting Ubx enhancer that contains several
hb DNA-binding sites and can direct an early Ubx-like pattern of
expression, the anterior boundary of which depends on repression by zygotic
hb activity. Perhaps the hb protein similarly defines the anterior boundaries
of expression of the remaining bithorax complex genes, abdominal-A
and abdominal-B, as suggested previously (White and Lehmann, 1986),
and, hence, constitutes the graded repressor initially proposed by Lewis
to control the differential activation of the bithorax complex (1978). The
hb gene was initially identified by Lewis as the Regulator of
postbithorax gene because he obtained mutations that interfered with
normal bithorax complex activity (Lewis, 1968). The existence of other unusual
hb alleles, which cause dramatic defects in Ubx expression
distinct from their effects on segmentation (White and Lehmann, 1986; Lehmann
and Nüsslein-Volhard, 1987), provides additional evidence that hb
protein interacts directly with the bithorax complex, instead of operating
solely through its action on Kr, kni, and gt .
Cascading Gradients
In wild-type embryos, the bcd gradient is thought to control most aspects
of head and thorasic pattern by directly regulating several subordinate
genes. However, in the abnormal situation in which maternal hb transcripts
are inactivated by mutation, the bcd gradient can direct the development
of abdominal, as well as head and thorasic pattern, even though its realm
of direct action is apparently limited to the anterior half of the body
(Hülskamp et al., 1989, 1990; Irish et al., 1989; Struhl, 1989a). As
shown here, it does so by activating high levels of a second gradient morphogen,
hb, at the extreme posterior limit of its effective range. The resulting
hb gradient in turn extends further posteriorly, triggering a series of
additional responses, one of which, the differential repression of gt,
generates a third gradient influencing yet more responses (e.g., the posterior
boundary of kni ; see above). Thus, bcd can organize the global pattern
by generating additional morphogen gradients that operate in regions outside
its immediate realm of action. The ability of bcd to organize the body plan
by triggering a series of such gradients may exemplify how a single, spatially
restricted morphogen can control global pattern.
Experimental Procedures
Control of the Posterior Kr and Anterior gt Boundaries by hb
Females of the following four genotypes were generated by conventional
genetic crosses:
bcdE1 hb FB
tsl035/bcdE1
tsl035
bcdE1 tsl035
HB547#4 hb547#6/CyO; bcdE1 tsl035
bcdE6 osk166
tsl035
Except where stated otherwise, only mutations directly relevant to the experiments are indicated (see Lindsley and Zimm, 1985, 1986, 1987, 1988 and references therein for descriptions of mutant alleles and balancers used). The bcdE6 mutation is an in-frame deletion of a portion of the homeodomain and acts as an amorph (Struhl et al., 1989). HB547#4 and HB547#6 are independent P element-mediated insertions of a 4.7 kb Bam fragment of hb genomic DNA into the second chromosome. This fragment contains genomic DNA including the hb coding sequence and its associated maternal and bcd-dependent regulatory sequences; it can rescue most aspects of the hb mutant phenotype, though it does not appear to include regulatory sequences relevant to activation of the hb gene under the control of the terminal system (Tautz et al., 1987; Schröder et al., 1988; Struhl et al., 1989; Hülskamp et al., 1989). In these experiments, the HB547 insertions behave as extra copies of the maternal hb gene function.
Embryos aged 0-4 hr after egg laying were obtained from females of each
genotype and fixed and stained in parallel for hb, gt, and Kr expression
using standard immunohistochemical procedures (Macdonald and Struhl, 1986;
see also below; rat ![]() -hb, rabbit
-hb, rabbit ![]() -Kr, and rabbit
-Kr, and rabbit ![]() -gt antisera were
provided by P. M. Macdonald, Ml. Levine, and V. Pirrotta, respectively).
When stained for hb expression, embryos derived from the first three genotypes
show a gradient of hb expression apparent as early as nuclear cycle
8 and persisting until the beginning of nuclear cycle 14 (stage 5(1); see
Lawrence and Johnston 11989] for staging during nuclear cycle 14). The concentration
of hb protein increases proportionally as the number of maternal
copies of the gene rises from 1 to 2 to 4(1x, 2x, and 4x in Figure 2; see
below). Embryos derived from bcdE6
osk166 tsl035
females show ubiquitous hb expression during the same period (osk
in Figure 2). These embryos were mixed, fixed, and stained together
with wild-type embryos (from which they can be distinguished by the absence
of pole cells) to control for the staining reaction. An example of such
a control can be seen in Figure 2, which shows Kr staining in a bcd osk
tsl-derived embryo (note the patterned expression of Kr in the neighb
oring wild-type embryo [lower right corner]).
-gt antisera were
provided by P. M. Macdonald, Ml. Levine, and V. Pirrotta, respectively).
When stained for hb expression, embryos derived from the first three genotypes
show a gradient of hb expression apparent as early as nuclear cycle
8 and persisting until the beginning of nuclear cycle 14 (stage 5(1); see
Lawrence and Johnston 11989] for staging during nuclear cycle 14). The concentration
of hb protein increases proportionally as the number of maternal
copies of the gene rises from 1 to 2 to 4(1x, 2x, and 4x in Figure 2; see
below). Embryos derived from bcdE6
osk166 tsl035
females show ubiquitous hb expression during the same period (osk
in Figure 2). These embryos were mixed, fixed, and stained together
with wild-type embryos (from which they can be distinguished by the absence
of pole cells) to control for the staining reaction. An example of such
a control can be seen in Figure 2, which shows Kr staining in a bcd osk
tsl-derived embryo (note the patterned expression of Kr in the neighb
oring wild-type embryo [lower right corner]).
We also find, unexpectedly, that hb is activated during the latter portion of nuclear cycle 14 (beginning during stage 5(2)) in embryos derived from females of the first three genotypes. This late expression occurs at the posterior pole and appears to depend on nos because it is absent in embryos derived from bcdE6 osk166 tsl035 females.
Although the concentration of hb protein can clearly be seen to be proportional to the maternal gene dosage in the anterior halves of 1x, 2x, and 4x embryos (e.g., Figure 2), it is not readily apparent from simple inspection that a similar proportional relationship exists in the posterior half of the body where hb protein expression is down-regulated by nos . To assess this quantitatively, we have directly measured the position at which hb protein expression falls beneath the level of detection in embryos obtained from females of each genotype (the measurements were performed using a Zeiss axioplan microscope [bright field optics] equipped with a graticule; 15 embryos in nuclear cycles 11 and 12 were scored for each genotype). We observed that the boundary shifted from 39% to 35% to 28% egg length (measured from the posterior pole) in 1x, 2x, and 4x embryos, respectively, indicating a proportional increase in the concentration of hb protein at any given position along the body. Because of the difficulties in recording low levels of protein expression photographically, these direct measurements provide a more accurate reflection of the extent of the hb protein gradient than that apparent in the optical cross sections shown in Figure 2. Note, however, that the hb gradient can be seen to extend approximately two-thirds of the way down the body in the wild-type embryo shown in Figure 1 (in which the plane of focus is on the surface of the embryo), consistent with our measurements.
Because the boundaries of Kr and gt protein expression are graded rather than sharp, we measured the position at which the amount of protein begins to decline from peak levels as well as the position at which it falls beneath the level of detection and recorded the halfway point in between. In general, both proteins fell from peak to undetectable levels of expression within 10% egg length. For each boundary determination given in the legend of Figure 2, we examined 12 embryos at stage 5(2).
To generate embryos completely lacking early hb protein expression as well as bcd and tor function (0x in Figure 2), chimeric females carrying torRX bcdE1 hbFB germ cells were obtained by pole cell transplantation. Agametic female host embryos were generated by crossing wild-type females to OvoD1/Y males. Donor embryos were obtained as the progeny of torRX bcdE1 hbFB/TM2, tor + males and females. The TM2, tor + balancer chromosome was obtained by P element-mediatod insertion of an 11-12 kb EcoRI fragment carrying the intact ttor + gene (Casanova and Struhl, 1989). The torRX bcdE1 hbFB mutant alleles were chosen because they appear to be protein nulls; identical results were also obtained in preliminary experiments using the torPM bcdE1 hb14F mutant alleles, which behave genetically as amorphs, although the torPMand hb14F alleles encode antigenically detectable protein products (Casanova and Struhl, 1989; Tautz, 1988). Chimeric OvoD1 /+ females carrying mutant germlines were initially identified because they laid eggs that did not hatch; the identification was then confirmed by mounting and inspecting pharate first instar larvae. Embryos derived from mutant germ cells differentiated a characteristic cuticular pattern of a large lawn of unpolarized abdominal denticles, followed posteriorly by a small lawn. This polarity in the cuticular pattern was unexpected because the only remaining determinant system operating in these embryos is nos. and its only known targets, bcdE1 hb14F maternal transcripts (Wharton and Struhl, 1989, 1991; Hülskamp et al., 1990), cannot encode protein. To test whether this polarity is generated by the action of nos on some other target molecule, we examined embryos derived f from chimeric females carrying torRX bcdE1 hbFB nosL7 germ cells (in this case, the donor embryos were obtained as the progeny of torRX bcdE1 hbFB nosL7/TM2, tor + males and females). These embryos differentiated a single lawn of unpolarized abdominal hairs similar to that formed by embryos derived from bcdE6 osk166 tsl035 females (Figure 4), confirming that the polarity observed in embryos 3 derived from torRX bcdE1 hbFB germ cells is nos- dependent.
Females carrying torRX bcdE1 hbFB germlines were then pooled, and their embryos were fixed and stained by immunohistochemical techniques for hb, Kr, and gt . As expected, these embryos do not express any hb protein up until nuclear cycle 14 (0x in Figure 2); however, as in the case of embryos derived from bcdE1 tsl035 females carrying between 1 and 4 copies of the hb gene, we find that hb is activated in the vicinity of the posterior pole during the latter portion of nuclear cycle 14 (beginning during stage 5(2)). This late expression may account for the polarized cuticular pattern formed by these embryos. To control for vagaries in fixation and staining, similarly aged embryos derived from bcd osk mutant females were included along with the experimental embryos throughout the fixation and staining procedure. These control embryos could be easily distinguished from the experimentals because they lacked pole cells.
Independent Control of the Posterior Kr and Anterior gt Expression
Boundaries
To test whether the hb gradient can define the posterior boundary of Kr
expression independent of gt gene activity, Df(1)62g18, gt -/+; bcdE1
tsl035 females were crossed to wild-type
males, and their progeny were double stained for Kr and gt protein expression
by immunohistochemistry as follows. After standard fixation, the embryos
were incubated in MeOH containing 3% H2O2 for 15 min (Kellerman et al., 1990), washed briefly
in MeOH, stained for Kr expression by standard immunohistochemical procedures
using a horseradish peroxidase-conjugated goat ![]() -rabbit antiserum and
the horseradish peroxidase signal developed using the conventional diaminobenzidine
color reaction, which generates an orange-brown signal. The embryos were
then incubated in 0.2 M glycine-HCl (pH 2.5) containing 0.1% Triton X-100
to strip embryos of the initial antiserum (Kellerman et al., 1990) and subjected
to a second round of antibody staining using rabbit
-rabbit antiserum and
the horseradish peroxidase signal developed using the conventional diaminobenzidine
color reaction, which generates an orange-brown signal. The embryos were
then incubated in 0.2 M glycine-HCl (pH 2.5) containing 0.1% Triton X-100
to strip embryos of the initial antiserum (Kellerman et al., 1990) and subjected
to a second round of antibody staining using rabbit ![]() -gt antiserum followed
by the horseradish peroxidase-conjugated goat
-gt antiserum followed
by the horseradish peroxidase-conjugated goat ![]() -rabbit antiserum. The
embryos were then stained again using the diaminobenzidine color reaction,
this time supplimented with nickel and cobalt ions (Lawrence and Johnson,
1989) to produce a blue-gray as opposed to orange color.
-rabbit antiserum. The
embryos were then stained again using the diaminobenzidine color reaction,
this time supplimented with nickel and cobalt ions (Lawrence and Johnson,
1989) to produce a blue-gray as opposed to orange color.
To test whether the hb gradient can define the anterior boundary of gt
expression independent of Kr gene activity, Kr1/+; bcdE1
and Kr1/+; bcdE6
osk166 females were generated by
standard genetic means, crossed to Kr1/+
males; their progeny were fixed, pooled together with wild-type embryos,
and double stained for gt and Kr protein expression by immunohistochemistry
as described above, except that gt staining was developed using the standard
horseradish peroxidase substrate to yield an orange-brown reaction product
and Kr staining was obtained using an alkaline phosphatase-conjugated goat
![]() -rabbit antiserum and the Vector Labs "black" substrate kit,
which generates a purple signal. The embryos shown in Figure 3 are representative,
except that we occasionally observed thin stipes of gt staining on either
side of the central Kr domain in Kr + embryos derived from bcdE1 oask166
females and on the anterior side of the central Kr domain in Kr+ embryos derived frombcdE1.
However, these thin stripes of gt staining were never observed in sibling
Kr+ embryos. We note that Kraut and
Levine (1991a) reported similar results to those obtained by us: they were
unable to detect gt expression in embryos derived from Kr1/+; nosL7
females outcrossed to Kr1/+ males,
even though one-quarter of the progeny should lack Kr gene activity.
However, in the absence of a positive control for gt staining, and, particularly,
given that these authors also report observing posterior gt expression in
a significant portion of Kr+ embryos
derived from nosL7 females in separate
experiments, we do not regard their negative results as compelling.
-rabbit antiserum and the Vector Labs "black" substrate kit,
which generates a purple signal. The embryos shown in Figure 3 are representative,
except that we occasionally observed thin stipes of gt staining on either
side of the central Kr domain in Kr + embryos derived from bcdE1 oask166
females and on the anterior side of the central Kr domain in Kr+ embryos derived frombcdE1.
However, these thin stripes of gt staining were never observed in sibling
Kr+ embryos. We note that Kraut and
Levine (1991a) reported similar results to those obtained by us: they were
unable to detect gt expression in embryos derived from Kr1/+; nosL7
females outcrossed to Kr1/+ males,
even though one-quarter of the progeny should lack Kr gene activity.
However, in the absence of a positive control for gt staining, and, particularly,
given that these authors also report observing posterior gt expression in
a significant portion of Kr+ embryos
derived from nosL7 females in separate
experiments, we do not regard their negative results as compelling.
Control of the Anterior kni Expression by hb
Females of the following five genotypes were generated by conventional genetic
crosses:
bcdE1hbFBtsl035/ bcdE1
tsl035.
bcdE1 tsl035.
HB547#4 HB547#6/CyO; bcdE1
tsl035.
bcdE6 osk166
tsl035.
HB547#4 HB547#6/CyO; bcdE6 osk166 tsl035.
Embryos derived from these females were fixed and stained in parallel
for kni protein expression as described above using a rat ![]() -kni antibody provided
by J. Dubnau. Cuticles of pharate first instar larvae were mounted for compound
microscopy in a mixture of 1:1 Hoyer's mountant:lactic acid (Struhl, 1984).
-kni antibody provided
by J. Dubnau. Cuticles of pharate first instar larvae were mounted for compound
microscopy in a mixture of 1:1 Hoyer's mountant:lactic acid (Struhl, 1984).
Control of the Anterior Expression of Kr Expression by bcd-Dependent
Zygotic Activity of hb
Females of the following three genotypes were prepared by standard genetic
crosses:
torRX; hbFB
nosL7/nosL7.
vasPDtorWKexuPJ.
vasPDtorWKexuPJ; hbFB/TM3,
hb-ß-gal.
The TM3, hb-ß-gal. balancer was obtained by using P element-mediated transformation to insert hb-ß-gal. fusion gene onto a conventional TM3, ri pPSb Ser e balancer; the hb-ß-gal. fusion gene consists of three copies of the bcd-dependent regulatory region of the hb gene (-260 to -60 relative to the hb transcriptional start; see Struhl et al., 1989) placed in front of an hsp70-ß-gal reporter gene, HZ50PL, which includes the hsp70 TATA box, but lacks the heat shock-dependent regulatory elements (Hiromi and Gehring, 1987). ß-gal expression derived from this gene is bcd dependent and can be detected by immunoreactivity as early as the beginning of nuclear cycle 14 (beginning of stage 5(1), approximately 60 min before the onset of gastrulation).
In the initial experiment (Figure 5), embryos aged 1-4 hr after egg laying
were obtained from orRX; hbFB nosL7/nosL7 females crossed to hbFB/+
males and then fixed and double stained for hb and Kr protein expression
according to standard immunofluorescence procedures (e.g., Macdonald and
Struhl, 1986); the rat ![]() -hb antiserum and rabbit
-hb antiserum and rabbit ![]() -Kr antiserum were
visualized using appropriate rhodamine and fluorescein secondary antibodies,
respectively. In subsequent experiments (Figure 6), similarly aged embryos
were derived from vasPDtorWKexuPJ; TM3,
hbFB/TM3, hb-ß-gal.
females crossed to hbFB/TM3, hb-ß-gal.
males. The embryos were then fixed and processed in parallel to detect both
ß-gal protein and either gt or Kr protein using the double-labeling
immunohistochemical procedures described above (the second signal was generated
using either a horseradish peroxidase-conjugatod secondary antibody followed
by the diaminobenzidine color reaction supplemented with nickel and cobalt
ions, or an alkaline phosphatase secondary antibody followed by the Vector
"black" substrate kit; similar results were obtained with both
staining protocols. A rabbit
-Kr antiserum were
visualized using appropriate rhodamine and fluorescein secondary antibodies,
respectively. In subsequent experiments (Figure 6), similarly aged embryos
were derived from vasPDtorWKexuPJ; TM3,
hbFB/TM3, hb-ß-gal.
females crossed to hbFB/TM3, hb-ß-gal.
males. The embryos were then fixed and processed in parallel to detect both
ß-gal protein and either gt or Kr protein using the double-labeling
immunohistochemical procedures described above (the second signal was generated
using either a horseradish peroxidase-conjugatod secondary antibody followed
by the diaminobenzidine color reaction supplemented with nickel and cobalt
ions, or an alkaline phosphatase secondary antibody followed by the Vector
"black" substrate kit; similar results were obtained with both
staining protocols. A rabbit ![]() hb-ß-gal antiserum from Cappel
was used to detect ß-gal protein expression.) In addition, sibling
embryos were stained for hb expression as described above.
hb-ß-gal antiserum from Cappel
was used to detect ß-gal protein expression.) In addition, sibling
embryos were stained for hb expression as described above.
Acknowledgments
We thank J. Casanova, J. Dubnau, K. Howard, and R. Wharton for discussion,
P. Fazio-Miceli and A. Nakanishi for technical assistance, J. Dubnau, M.
Levine, P. M. Macdonald, and V. Pirona for antibodies, W. Driever, M. Hülskamp,
V. Pirrotta, T. Schupbach, and D. Tautz for stocks, and R. Axel, J. Dubnau,
T. Jessell, and R. Wharton for comments on the manuscript. This work was
supported by Howard Hughes Medical Institute, the McKnight Foundation for
Neuroscience, and the Medical Research Council of Great Britain.
The costs of publication of this article were defrayed in part by the payment
of page charges. This article must therefore be hereby marked "advertisement"
in accordance with 18 USC Section 1734 solely to indicate this fact.
Received October 15, 1991; revised February 6, 1992.
References
Berleth, T., Burri, M., Thoma, G., Bopp, D., Richstein, S., Frigerio, G., Noll, M., and Nüsslein-Volhard, C. (1988). The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J . 7, 1749-1756.
Carroll, S. B., and Scott, M. P. (1986). Zygotically active genes that affect the spatial expression of the fushi tarazu segmentation gene during early Drosophila embryogenesis. Cell 45, 113-126.
Casanova, J ., and Struhl, G . (1989). Localized surface activity of torso, a receptor tyrosine kinase, specifies terminal body pattern in Drosophila. Genes Dev. 3, 2025-2038.
Cofen, S. M., and Jurgens, G. (1990). Gap-like segmentation genes that mediate Drosophila head development. Nature 346, 482-485.
Daloq, A. M. (1938). Form and Causality in Early Development (Cambridge, England: Cambridge University Press).
Dalton, D., Chadwick, R., and McGinnis, W. (1989). Expression and embryonic function of empty spiracles: a Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev. 3, 1940-1956.
Driever, W., and Nüsslein-Volhard, C. (1988). The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54, 95-104.
Driever, W., and Nüsslein-Volhard, C. (1989). The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 337, 138-143.
Driever, W., Thoma, G., and Nüsslein-Volhard, C. (1989). Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature 340, 363-367.
Eldon, E . D., and Pirrotta, V . (1991). Interactions of the Drosophila gap gene giant with maternal and zygotic pattern-forming genes. Development 111, 367-378.
Finkelstein, R., and Perrimon, N. (1990). The orthodenticle gene is regulated by bicoid and torso and specifies Drosophila head development. Nature 346, 485-488.
Frasch, M., and Levine, M. (1987). Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev.1, 981-995.
Frohnhöfer, H. G., and Nüsslein-Volhard, C. (1987). Maternal genes required for the anterior localization of bicoid activity in the embryo of Drosophila. Genes Dev. 1, 880-890.
Gaul, U., and Jäckle, H. (1987). Pole region-dependent repression of the Drosophila gap gene Krüppel by maternal gene products. Cell 51, 549-555.
Gaul, U., and Jäckle, H. (1989). Analysis of maternal effect mutant combinations elucidates regulation and function of the overlap of hunchback and Krüppel gene expression in the Drosophila blastoderm embryo. Development 107, 651-662.
Hiromi, Y., and Gehring, W. J. (1987). Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell 50, 963-974.
Hoch, M., Seifert, E., and Jäckle, H. (1991). Gene expression mediated by cis-acting sequences of the Krüppel gene in response to the Drosophila morphogens bicoid and hunchback. EMBO J.10,2267-2278.
Hülskamp, M., Schröder, C., Pfeifle, C., Jäckle, H., and Tautz, D. (1989). Posterior segmentation of the Drosophila embryo in the absence of a maternal posterior organizer gene. Nature 338, 629-632.
Hülskamp, M., Pfeifle, C., and Tautz, D. (1990). A morphogenetic gradient of hunchback protein organizes the expression of the gap genes Krüppel and knirps in the early Drosophila embryo. Nature 346, 577580.
Ingham, P. W., Ish-Horowicz, D., and Howard, K. (1986). Correlative changes in homeotic and segmentation gene expression in Krüppel mutant embryos of Drosophila. EMB0 J. 5, 1659-1665.
Irish, V., Lehmann, R., and Akam, M. (1989). The Drosophila posterior group gene nanos functions by repressing hunchback activity. Nature 335, 646-648.
Jäckle, H., Tautz, D., Schuh, R., Seifert, E., and Lehmann, R. (1986). Cross regulatory interactions among the gap genes of Drosophila. Nature 324, 668-670.
Kellerman, K. A., Moattson, D. M., and Duncan, l. (1990). Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes Dev. 4, 1936-1950.
Knipple, D. C., Seifert, E., Rosenberg, U. B., Preiss, A., and J§Jäckle, H. (1985). Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature 317, 40-44.
Kraut, R., and Levine, M. (1991a). Spatial regulation of the gap gene giant during Drosophila development. Development 111, 601 -609.
Kraut, R., and Levine, M. (1991b). Mutually repressive interactions between the gap genes giant and Krüppel define middle body regions of the Drosophila embryo. Development 111, 611-621.
Lawrence, P. A., and Johnston, P. (1989). Pattern formation in the Drosophila embryo: allocation of cells to parasegments by even-skipped and fushi tarazu. Development 105, 761-767.
Lehmann, R., and Nüsslein-Volhard, C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141-152.
Lehmann, R., and Nüsslein-Volhard, C. (1987). hunchback, a gene required for segmentation of an anterior and posterior region of the Drosophila embryo. Dev. Biol. 119, 402-417.
Lehmann, R., and N;Nüsslein-Volhard, C. (1991). The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development 112, 679-693.
Lewis, E. B. (1968). Genetic control of developmental pathways in Drosophila melanogaster. Proc. 12th. Int. Congr. Genetics 2, 96-97.
Lewis, E. B. (1978). A gene complex controlling segmentation in Drosophila. Nature 276, 565-570.
Lindsley, D., and Zimm, G. (1985). The Genome of Drosophila melanogaster. Dros. Inf. Serv. 62.
Lindsley, D., and Zimm, G. (1986). The Genome of Drosophila melanogaster. Dros. Inf. Serv. 64.
Lindsley, D., and Zimm, G. (1987). The Genome of Drosophila melanogaster. Dros. Inf. Serv. 65.
Lindsley, D., and Zimm, G. (1988). The Genome of Drosophila melanogaster. Dros. Inf. Serv. 68.
Macdonald, P., and Struhl, G. (1986). A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature 324, 537-545.
Nauber, U., Pankratz, M. J., Kienlin, A., Seifert, E., Klemm, U., and Jäckle, H. (1988). Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps . Nature 336, 489-492.
Nüsslein-Volhard, C. (1991). Determination of the embryonic axes of Drosophila. Development (Suppl.) 1, 1-10.
Nüsslein-Volhard, C., and Wieschaus, E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801.
Nüsslein-Volhard, C., Frohnhöfer, H. G., and Lehmann, R. (1987). Determination of anteroposterior polarity in Drosophila. Science 238, 1675-1681.
Pankratz, M. J., Hoch, M., Seifert, E., and Jäckle, H. (1989). Krüppel requirement for knirps enhancement reflects overlapping gap gene activities in the Drosophila embryo. Nature 341, 337-340.
Qian, S., Capoviila, M., and Pirronas V. (1991).The bx region enhancer, a distant cis-control element of the Drosophila Ubx gene and its regulation by hunchback and other segmentation genes. EMBO J.10,14151426.
Sander, K. (1959). Analyse des ooplasmatischen Reaktionssystems von Euscelisplebejus Fall. (Cicadina) durch Isolieren und Kombinieren von Keimteilen. i. Mitt.: Die Differenzierungsleistungen vorderer und hinterer Eiteile. Rouz' Arch. Entwicklungsmech. Org. 151, 430-497.
Sander, K. (1960). Analyse des ooplasmatischen Reaktionssystems von Euscelisplebejus Fall. (Cicadina) durch Isolieren und Kombinieren von Keimteilen. ll. Min.: Die Differenzierungsleistungen nach Verlagern von Hinterpolmaterial. Rouz' Arch. Entwicklungsmech. Org. 151, 660-707.
Sander, K. (1975). Pattern specification in the insect embryo. Cell patterning. Ciba Foundation Symp. 29, 241-263.
Schröder, C., Tautz, D., Seifert, E., and Jäckle, H. (1988). Differential regulation of the two transcripts from the Drosophila gap segmentation gene hunchback. EMBO J. 7, 2882-2887.
Small, S., Kraut, R., Hoey, T., Warrior, R., and Levine, M. (1991). Transcriptional regulation of a pair-rule stripe in Drosophila. Genes Dev. 5, 827-839.
Stanojevic, D., Hoey, T., and Levine, M. (1989). Sequence-specific DNA binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature 341, 331-335.
Struhl, G. (1981). A gene product required for correct initiation of segmental determination in Drosophila. Nature 293, 36-41.
Struhl, G. (1984). Splitting the bithorax complex of Drosophila. Nature 308, 454-457.
Struhl, G. (1989a). Differing strategies for organizing anterior and posterior body pattern in Drosophila embryos. Nature 338, 741-744.
Struhl, G. (1989b). Morphogen gradients and the control of body pattern in insect embryos. The cellular basis of morphogenesis. Ciba Foundation Symp. 144, 65-86.
Struhl, G., Struhl, K., and Macdonald, P. M. (1989). The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell 57, 1259-1273.
Tautz, D. (1988). Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centers. Nature 332, 281-284.
Tautz, D., Lehmann, R., Schnurch, H., Schuh, R., Seifert, E., Kienlin, A., Jones, K., and Jäckle, J. (1987). Finger protein of novel structure encoded by hunchback, a second member of the gap class of Drosophila/a segmentation genes. Nature 327, 383-389.
Treisman, J., and Desplan, C. (1989). The products of the Drosophila gap genes hunchback and Krüppel bind to the hunchback promoters. Nature 341, 335-337.
Turing, A. (1952). The chemical basis of morphogenesis. Phil. Trans, Roy. Soc. (Lond.) B 237, 37-72.
von Ubisch, L. (1953). Entwicklungsprobleme (Jena, Germany: Gustav Fischer Verlag).
Wang, C., and Lehmann, R. (1991). nanos is the localized posterior determinant in Drosophila. Cell 66, 637 647.
Wharton, R. P., and Struhl, G. (1989). Structure of the Drosophila BicaudalD protein and its role in localizing the posterior determinant nanos . Cell 59, 881-892.
Wharton, R. P., and Struhl, G. (1991). RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos . Cell 67, 955-967.
White, R. A. H., and Lehmann, R. (1986). A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell 47, 311-321.
Return To Molecular Biology Main Page
© Copyright
2000 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu