Introduction
Muscle contraction occurs when calcium ions bind to the troponin/tropomyosin complex, changing its shape so that the binding sites on actin are exposed. In adult muscle cells, the calcium concentration in the cytoplasm is controlled by the sarcoplasmic reticulum (SR). The SR membrane contains calcium pumps which transport calcium ions from the cytoplasm into the lumen so that the fibrils can relax. The SR releases calcium via calcium channels into the cytoplasm so that the fibrils can contract. Maintenance of the calcium concentration within the SR is accomplished by calcium buffers, calcium channels and calcium pumps (fig 1). During muscle relaxation, the calcium release channels of the SR are closed and the calcium-ATPases pump calcium ions from the cytosol into the lumen. During muscle contraction, the calcium release channels are opened and calcium is released from the lumen into the cytosol (Krause et. al, 1990). A calcium buffer is contained within the lumen of the SR to reduce the concentration gradient which the calcium pumps must work against, and to prevent the precipitation of calcium salts inside the SR. The major calcium buffer in muscle cells is calsequestrin and it is the key to successful storage and release of calcium (Tharin et. al, 1992).
The recently cloned rabbit cardiac calsequestrin gene is over 30 kb and
contains 11 exons. It also includes 1198 bp of 3'-untranslated, 85bp of 5'-untranslated,
and a single poly-A addition site (Arai et. al, 1991). The exon boundaries are located
at the predicted beginnings and ends of helixes and the sequences which encode ß
sheets are located within exons. All isoforms of the calsequestrin gene have similar
splicing patterns, so no alternative splicing pattern is apparent (Yano and Zarain
Hertzberg, 1994).
Calsequestrin is an anchored protein network within the lumen of the SR
(Yano and Zarain-Hertzberg, 1994) that is retained within the lumen of the SR after
transcription. The protein includes a signal sequence in its first 20 amino acids, that
targets the protein to the SR, which is immediately cleaved off the functional
protein after translation. The protein does not include KDEL, the amino acid
sequence which has been shown to cause a protein to remain in the ER (Munro and
Pellum, 1987). Since there is no apparent mechanism to keep the calsequestrin
inside the SR, it must either use an unknown mechanism or bind to another SR
protein.
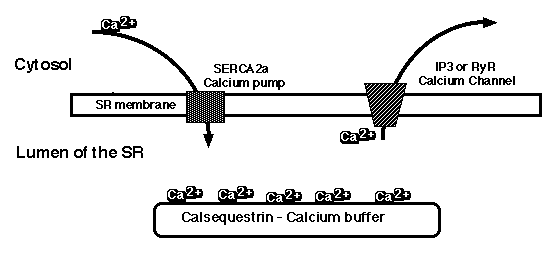
Figure 1: Depiction of the calcium pool in cardiac sarcoplasmic reticulum. The calcium ions are actively pumped into the SR by SERCA2a, bound in the lumen by calsequestrin, and released into the cytosol by IP3- or ryanodine receptors. This movement of calcium ions across the SR membrane is
necessary for muscle contraction and relaxation.
Calsequestrin is not a transmembrane protein, and it is very hydrophilic (fig 2). The protein is also highly acidic and has a calculated isoelectric point of 4.01 (Arai et. al, 1991). The protein has a large proportion of negatively charged amino acids, which are thought to be responsible for binding calcium. The highly acidic carboxyl- half contains multiple aspartic acid residues, suggesting it is the most structured region of the protein and an important component for calcium binding (Yano and Zarain-Hertzberg, 1994) (fig 3). Also, calsequestrins from different species vary mainly in the carboxyl terminus (fig 3). This observation suggests that the contrasting lengths of the carboxyl termini may designate the amount of calcium needed inside the SR for each species (Yano and Zarain-Hertzberg, 1994).
Figure 2: Kyte and Doolittle hydrophobicity plot of rabbit cardiac calsequestrin. No region of the protein is above 2.0 (except signal sequence) which shows that calsequestrin is a very hydrophilic protein without a transmembrane portion. X-axis denotes amino acid residues and Y-axis denotes relative hydrophobicity.
Figure 3: Aligned amino acid sequences of all cloned skeletal and cardiac calsequestrins. Shaded amino acids indicate identical amino acid residues between sequences. Notice the high content of apartic acid (D) in the carboxyl-terminus of each sequence.
The secondary structure of calsequestrin is mainly helical and the carboxyl half consists of some ß-sheet structure. The tertiary and quaternary structure forms the network characteristic of the protein. Calsequestrin contains apparent binding sites for other proteins, which may allow interactions with the calcium pump and the calcium channel proteins (Krause et. al, 1990). Calsequestrin has an estimated molecular weight of approximately 43 kD.
The calcium buffer, calsequestrin, is a low-affinity (kD 100µM), high-capacity (up to 45 moles calcium/ mole of calsequestrin) calcium-binding glycoprotein. When there is an influx of calcium into the SR, calsequestrin can bind large amounts of calcium due to its high capacity. When the release channels are opened, the calcium can quickly dissociate from the calsequestrin, due to its low affinity, and move into the cytoplasm by diffusion. The function of the calcium buffer is also to reduce the calcium gradient across the membrane and to keep the calcium ions from precipitating within the lumen.
Calsequestrin is a highly conserved protein and shares up to 90% homology
in different species (fig 4). In the phylogenic tree in figure 4, rabbit cardiac
calsequestrin is indicated to share more homology to other cardiac calsequestrins
than the rabbit skeletal calsequestrin. This comparison shows that the cardiac
isoform is more similar to calsequestrins with similar function than to different
isoforms within the same species. Interestingly, chicken skeletal calsequestrin is
ranked as more homologous to cardiac calsequestrins than other skeletal
calsequestrins. This raises a question of the difference in avian evolution of calcium
proteins compared to other vertebrates.
Figure 4: Phylogenetic tree of all cloned calsequestrins. The sequences have been ranked by homology and the percentages represent the portion of similar amino acids between the proteins.
Calsequestrin is included in a family of proteins with similar function but varied primary structure. The biochemical properties which define a protein as a member of the calsequestrin family include: high-capacity, low-affinity calcium binding; metachromatic blue staining with the carbocyanine dye Stains-all; change of apparent molecular mass with pH; and high proportion of acidic amino acids. Other less commonly applied criteria are: solubility in ammonium sulfate; pH and calcium-dependent precipitation; and decreased hydrophobicity at high calcium concentrations. (Krause et. al, 1990).
As mentioned above, two calsequestrin isoforms have been detected in mammals. One is expressed in cardiac and slow-twitch skeletal muscle (cardiac isoform), while the other is expressed in fast-twitch and slow-twitch skeletal muscle (fast-twitch isoform). These two isoforms are encoded by separate genes, but have similar properties and 65% amino acid identity (Choi and Clegg, 1990). The primary structure for both proteins has been determined through cDNA cloning.
Each type of muscle uniquely expresses the two isoforms of calsequestrin during muscle development. Cardiac muscle expresses only the cardiac isoform and the mRNA increases with development. Fast-twitch skeletal muscle coexpresses the cardiac and fast-twitch isoform in fetal and neonatal stages. The cardiac isoform mRNA level gradually decreases during development and is undetectable in adult fast-twitch skeletal muscle. The fast-twitch isoform mRNA increases and eventually replaces the cardiac isoform in adult fast-twitch muscle, indicating a switch in isoforms during development. This occurrence suggests that the two genes may share some common regulatory mechanisms (Arai et. al, 1991). In adult slow-twitch skeletal muscle, the fast-twitch isoform mRNA is strongly expressed while the cardiac isoform mRNA is barely detectable (Arai et. al, 1991).
The heart is the first working organ in the chicken embryo. The initial heart beat occurs between Hamburger and Hamilton development stages 9 - 10 (1951), before the SR calcium pool exists. The first coordinated contractions are not localized in any area, but occur in an unpredictable sequence along the right ventricular wall (Patten and Kramer, 1949). The expression of the calcium pool components during development in relation to the initial cardiac contraction has not been determined.
Experimentation with early chicken embryos has determined that when
calcium channels in the plasma membrane are blocked, early heart contractions do
not occur (Renaud et. al, 1984; Hasin and Barry, 1987; Sakai et. al, 1983). Therefore,
initial contractions of cardiac myocytes rely on the influx of extracellular calcium.
There is a developmental switch from the use of extracellular calcium for muscle
contraction to the use of intracellular calcium regulated by the SR, but it has not
been determined when or how this switch occurs. To understand the mechanism
and timing of this switch, it is important to determine the developmental stage and
location of initial calsequestrin expression to know when the calcium is first
buffered in the lumen of the SR.
Materials and Methods
Fertilized chicken eggs (provided by Hubbard Farms, Statesville, NC) were incubated for the desired time and staged upon being removed from the shell. Staging was done according the characteristics described by Hamburger and Hamilton (1951).
The older chicken embryos (stages 19 - 21) were prepared by cryostat sectioning at 12µm thickness. The frozen sections were melted directly onto a slide and a circle of rubber cement was put around the section to limit reagent volumes. The tissues were hydrated with phosphate bovine solution (PBS - 1% NaCl, 0.025% KCl, 0.18% Na2HPO4, 0.03% KH2PO4). Next, the tissues were hydrated with 1% bovine serum albumin (BSA) PBS solution for 5 minutes. The sections were then fixed in PBS with 4% paraformaldehyde for 5 minutes and left in PBS, 1% BSA and 5 mg/ml lysine for 5 minutes. The primary antibody (polyclonal rabbit antisera to canine cardiac calsequestrin; Jorgensen and Campbell, 1984) was added in PBS, 1% BSA and 0.25% saponin and incubated for 45 minutes at room temperature. This antibody was generously provided by Dr. Kevin Campbell at the University of Iowa. The sections were washed 3 times with PBS and the secondary antibody (FITC conjugated goat anti-rabbit, purchased from KPL, Gaithersburg, MD) in PBS, 1% BSA and 0.25% saponin was added and incubated for 45 minutes at room temperature. The sections were washed three times again, covered in mounting medium (90% glycerol, 10% PBS, 0.1% para-phenylenediamine) and sealed with a cover slip and clear fingernail polish. The slide was then photographed to observe labeling.
The younger embryos (stages 12 - 19) were whole-mounted on slides. The eggs were cracked into petri dishes and the embryo was rolled to the top of the yolk. A donut-shaped ring of 42.5mm Whatman #3 filterpaper was placed on top of the embryo. While holding the ring with forceps, the membranes on the margin of the paper were cut away. The paper was carefully lifted, with the embryo, and put into PBS to remove excess yolk. The embryos were emersed into buffered 4% paraformaldehyde for 10 minutes at room temperature, then transferred into blocking solution (BSA and 5 mg/ml lysine) for 5 minutes at room temperature. The embryo was placed on the slide, yolk side down, and rubber cement was added around the edges. The slide was put into a humid chamber and the primary antibody in BSA and saponin was added to each slide. The embryos were incubated overnight at 4° C. The next day, the antibody was removed and the embryo was washed throughout the day with three changes of buffer while being maintained at 4° C. At the end of the day, the embryo was covered with secondary antibody and incubated overnight. The antibody was removed and the embryos were washed with three changes of buffer the next day, covered with mounting medium, sealed with a coverslip, and photographed the embryo.
All slides were viewed with an epifluorescence microscope. They were
photographed with T-max 400 35mm film (Kodak) and developed according to the
manufacture's directions.
Results
For a positive control, embryos were labeled with a calcium-ATPase antibody, an antibody used in previous experiments which produced positive results. These embryos showed thorough and consistent fluorescence while the embryos labeled with only secondary antibody, which served as a negative control, showed no fluorescence (data not shown). Cryosections of adult heart labeled with the calsequestrin antibody and fluorescent secondary antibody showed labeling similar in brightness and pattern to the labeling of adult cardiac muscle in Jorgenson and Campbell (1991) (fig 5). The fluorescence under the microscope appeared green, but appears white in the black and white photographs.
Figure 5: Adult chicken heart muscle labeled with the calsequestrin antibody and fluorescent secondary antibody. 10X objective lens.
The cryosections of stage 19 cardiac tissue was thoroughly labeled throughout the heart (fig 6); similar to adult heart labeling. Stage 17 embryo hearts were completely labeled (fig 7), and the cellular structure can be detected at higher magnification (fig 8). The subcellular labeling of cells, visible with the 10x objective lens (fig 8), shows a dark nucleus and fluorescent S/ER in the surrounding cytoplasm. In stage 14 embryos, only a few cells in one area of the heart actually labeled. The labeled cells are located in the section of the heart which later becomes the atrium, suggesting that calsequestrin first appears in the atrium of the chicken embryo heart (fig 9). Younger stages showed no labeling (data not shown).
Figure 6: Stage 19 embryo heart labeled with the calsequestrin antibody and the fluorescent secondary antibody. 10X objective lens.
Figure 7: Stage 17 embryo heart labeled with the calsequestrin antibody and the fluorescent secondary antibody. 3.2X objective lens.
Figure 8: Stage 17 embryo heart labeled with the calsequestrin antibody and the fluorescent secondary antibody. 10X objective lens.
Figure 9: Stage 14 embryo heart labeled with the calsequestrin antibody and the fluorescent secondary antibody. These labeled cells are in the section of cardiac tissue which will become the atria of the heart. 10X objective lens.
Discussion
According to the results of this study, calsequestrin appears in chicken embryos at stage 14 in the presumptive atrium and later is present throughout the entire heart by stage 17. Since the first contraction of the heart occurs between stages 9 and 10, calsequestrin is first expressed after contractions have begun. Also, the first contractions occur in the presumptive ventricle (Patten and Kramer, 1949) while calsequestrin first appears in the atrium. However, by stage 14, the atrium is also contracting and is acting as the pace maker for the entire heart (Patten and Kramer, 1949). Studies on the other components of the SR calcium pool in chicken embryo hearts have determined that the calcium pump (SERCA2) first appears at stage 10, around the time of the first contractions, and is expressed throughout the heart by stage 14 (Mercer and Cromartie, personal communication). Also, the calcium pump is initially expressed along the right margin on the heart. The initial contractions of the heart also begin along the right margin, or the presumptive ventricle. The IP3 receptor calcium channels have been shown to be present by stage 21 (Cromartie, personal communication). However, ryanodine receptors, which are known to be the calcium channels present in adult muscle tissue, have not been detected because the antibody used was unknowingly specific for the chicken skeletal isoform only (personal communication).
Calsequestrin may not be the only calcium binding protein in the lumen of the SR in muscle cells. Calreticulin, a functional analogue of calsequestrin, is a constituent calcium-binding protein in cardiac and skeletal muscle. Calreticulin is expressed in early fetal rat skeletal and cardiac muscle, but is down regulated during development. Calsequestrin appears later in development and becomes the major calcium binding protein and plays a major role in SR maturation (Imanaka-Yoshida et. al, 1996). In future experiments, chicken embryos should be labeled for calreticulin to determine if and when it appears in development of the heart.
Muscle development is thought to require a network of interdependent, temporally regulated gene activation. Protein expression during muscle differentiation is known to induce expression of later proteins. Little is known about the formation of SR, but calsequestrin immunoreactivity is detected in myoblasts before the appearance of recognizable SR (Choi and Clegg, 1990). After the formation of the SR, skeletal calsequestrin immunoreactivity takes on a fibrous appearance, similar to that of newly formed SR, suggesting that calsequestrin may initiate SR formation (Choi and Clegg, 1990). However, each muscle type expresses a unique program of SR proteins during muscle development. Cardiac SR development relies on the abundance of a single calsequestrin isoform, not two distinct isoforms as in skeletal muscle (Yano and Zarain-Hertzberg, 1994). SR gene transcripts are coordinately induced during muscle differentiation (Arai et. al, 1991). Contractile protein mRNA and SR gene transcripts appear simultaneously during skeletal muscle differentiation, indicating the coordination of their expression. The SR gene transcripts appear just before myotube formation and then simultaneously with contractile protein mRNA. These observations suggest that the activation of SR genes is under the control of muscle development differentiation regulators, such as the myoD gene family (Arai et. al, 1991).
However, in this research it was shown that SR transcripts are not
coordinately induced. Each SR calcium pool protein is initially expressed at a
different time and location, contradicting the idea that all SR proteins appear
simultaneously during development. In this study, calsequestrin has been found to
appear later in development than the calcium pump, but before the appearance of
IP3 receptors. From this information, we can hypothesize what event in SR
development turns on calsequestrin expression. Since the calcium pumps are
present in the early SR, perhaps the build up of calcium ions, or the flow of calcium
across the SR membrane, triggers calsequestrin transcription. Until the timing of
ryanodine receptor calcium channel expression is determined, we will not know if
the calcium is allowed to move out of the primitive SR when calsequestrin first
appears. Future research should include detecting for ryanodine receptors in early
embryos, as well as calreticulin, to further our knowledge of SR development.
References
Campbell A. M., P.D. Kessler, Y. Sagara, G. Inesi and D. M. Fambrough. Nucleotide sequences of avain cardiac and brain SR/ER Ca2+- ATPases and functional comparisons with fast twitch Ca2+-ATPases. J. Of Biol. Chem. 266: 16050 16055, 1991.
Choi, E. S. And D. Clegg. Identification and developmental expression of a chicken calsequestrin homolog. Developmental Biology. 142: 169-177, 1990.
Hamburger,V. and V. Hamilton. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49-92, 1951.
Hasin, Y. And W. H. Barry. Comparison of simultaneous electrophysiologic and mechanical effects of verapamil and nifedipine in cultured chick embryo ventricular cells. J. Mol. Cell Cardiol. 19:853-863, 1987.
Imanaka-Yoshida, K., A. Amitanti, S. Ioshii, S. Koyabu, T. Yamakado, T. Yoshida. Alterations of expression and distribution of the calcium storing proteins in endo/sarcoplasmic reticulum during differentiation of rat cardiomyocytes. J. of Molecular and Cellular Cardiology. 28: 553-562, 1996.
Jorgensen, A. and K. Campbell. Evidence for the presence of calsequestrin in two structurally different regoins of myocardial sarcoplasmic reticulum. J. Of Cell Biology . 98: 1597-1602, 1984.
Krause, K., K. Campbell, M. J. Welsh and D. P. Lew. The calcium signal and neutrophil activation. Clinical Biochemistry. 23: 159-166, 1990.
Patten, B.M. And T. C. Kramer. Initiation and early changes in the character of the
heart beat in vertebrate embryos. Physiol. Rev. 29: 31-47, 1949.
Munro, Sean and Hugh R. B. Pellum. A C-terminal signal prevents secretion of
luminal ER proteins. Cell. 48: 899-907, 1987.
Renaud, J. F., T. Kazazoglou, A. Schmid, G. Romey and M. Lazdunski. Differentiation of receptor sites for [3H]nitrendipine in chick hearts and physiological relation to the slow Ca2+ channel and to excitation-contraction coupling. Eur. J. Biochem. 139: 673-681, 1984.
Sakai, T., S. Fujii, A. Hirota and K. Kamino. Optical evidence for calcium-action potentials using early embrionic precontractile chick heart using a potential sensitive dye. Membrane Biol. 72: 205-212, 1983.
Tharin, S., E. Dziak, M. Michalak and M. Opas. Widespread tissue distribution of rabbit calreticulin, a non-muscle functional analogue of calsequestrin. Cell and Tissue Research. 269: 29-37, 1992.
Yano, K., A. Zarain-Hertzberg. Sarcoplasmic reticulum calsequestrins: structural
and functional properties. Molecular Cell Biochem. 135(1): 161-170, 1994.
Return to Biology Students' Home Page
Return to Davidson College Biology Department Home Page
© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to:
macampbell@davidson.edu