Purification and Characterization of Isocitrate Dehydrogenase
from Brassica oleracea
Rudy Beiler, Leo Dozier, Brad Edwards, Slade Worley, and John H. Williamson
Biology Department, Davidson College, Davidson, NC 28036-1719 USA
ABSTRACT
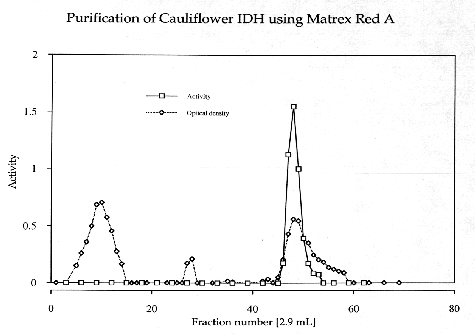
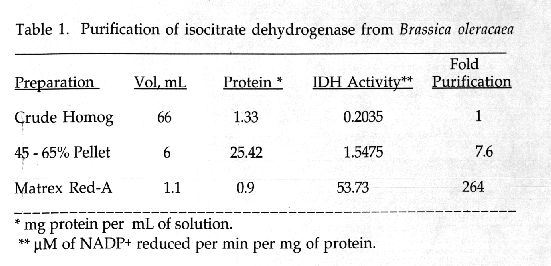
NADP-dependent isocitrate dehydrogenase was purified from Brassica oleracea (cauliflower) using ammonium sulfate precipitation, Matrex Red A affinity chromatography and Sephacryl S-300 gel filtration.
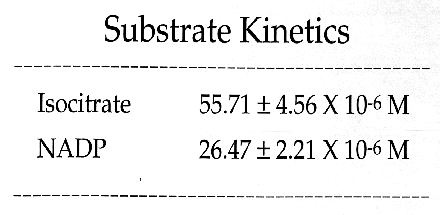
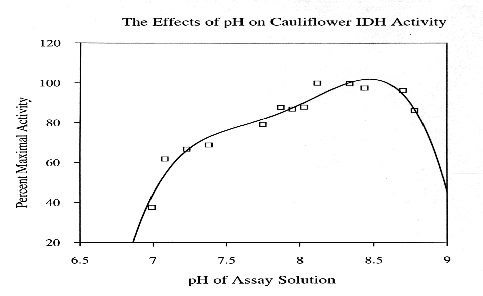
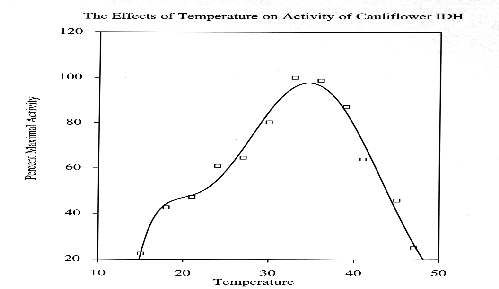
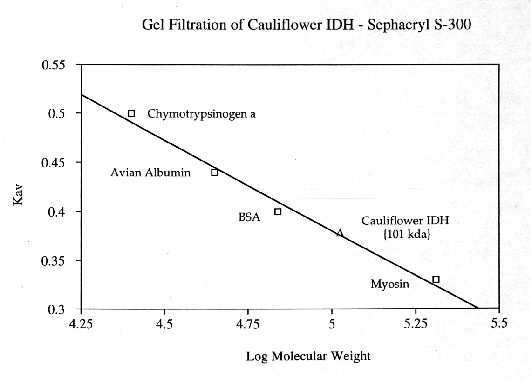
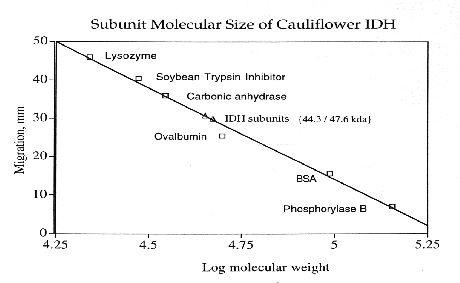
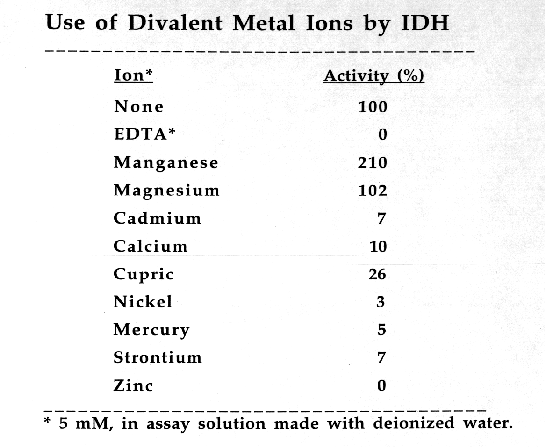
Cauliflower IDH is a dimeric enzyme with a native molecular size of approximately 100 kda and a subunit size of approximately 47 kda. Km values for DL-isocitrate and NADP were 55.71 +/- 4.56 X 10(-6) M and 26.47 +/- 2.21 X 10(-6) M, respectively. In assay solutions made with deionized water, enzyme activity appears to be independent of divalent metal activity. However, in the presence of 5 mM EDTA the enzyme appears to have an absolute requirement for divalent metal ions. Activity is enhanced by the addition of 5 mM Mn+2 but is not affected by 5mM Mg+2. Ca+2, Cd+2, Cu+2, Hg+2, Ni+2, Sr+2, and Zn+2 ions (at 5mM)are toxic to IDH activity. Cauliflower IDH is relatively heat sensitive, denaturing above 35-37 degrees C. The enzyme has maximal activity at pH's around 8.3 to 8.8.

© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to: jowilliamson@davidson.edu