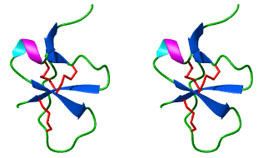
| Torres et al (1999) also determined
that the tertiary protein structure of DLP-1 (Fig. 6) is similar to the
protein structure of ShI, a sea anemone toxin. This similarity in peptide
shape is due to the common disulphide-connectivity pattern between DLPs
and ShI. However, experiments show that DLPs do not function in the same
manner as ShI, which is known to interfere with sodium channel activity
in ganglion (nerve) cells. This difference in biological function could
be due to the fact that the amino acid sequence of DLPs and ShI bear little
resemblance (Torres et al., 1999). Although DLPs do not share the same
biological function as b-defensin-12 or ShI, the similar tertiary shape
of all three proteins suggests that this distinct structural peptide core
has evolved as an effective way to yield small compact molecule displaying
many pharmological and physiological activities (Torres et al., 2000).
The biological role of DLPs in
platypus venom is yet to be determined. One possible explanation is that
the DLPs act synergistically with nerve growth factor (NGF) to produce
pain, the prominent symptom of platypus envenomation. NGF has been shown
to increase pain sensitivity in humans for three to four weeks. The nature
and time course of these symptoms bear a striking resemblance to the reported
symptoms of platypus envenomation (de Plater et al., 2001). However, more
research must be done on these novel components of platypus venom before
a definitive understanding of their pharmacology can be attained.
|