

What is the purpose of
microdissection?
Microdissection is
essential when analyzing tissue samples because the desired cells are sometimes
only a fraction of the total tissue sample. Microdissection allows
the desired tissue or cells to be separated from the rest of the
sample for molecular analysis. LCM is a specialized form of microdissection
that allows the researcher or pathologist to automatically perform this
technique under a microscope by simply pushing a button. This allows
for easier analysis of tissue samples, by isolating them from the surrounding
cells, so that the desired tissue can be analyzed to examine changes in
gene expressions or cellular DNA that are associated with different transitional
stages of certain diseases. This method allows the researcher to
detect the progression of cancer and other diseases without contaminating
the rest of the tissue sample3.


How is LCM performed?
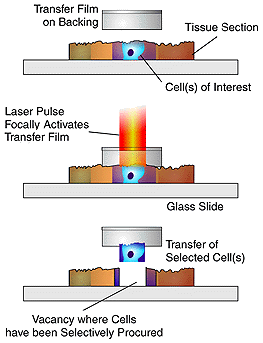
LCM uses a laser beam
and transfer film to lift the desired cells out of the tissue sample, separating
the unwanted cells and tissue from the sample for analysis. The transparent
transfer film is placed on the surface of the tissue sample, and a using
a microscope the researcher selects a group of cells. The researcher
uses a joystick to manuever the sample until the desired group of cell
is in the center of the field of view. Once the sample is centered,
the operator pushes a button to activate a laser diode in the microscope
optics. The laser diode produces a pulsed laser beam that is directed
onto the transfer film directly above the desired cluster of cells.
The laser melts the transfer film, fusing the cells with the film.
When the film is removed from the tissue sample the desired cells are taken
with it, leaving behind unwanted tissue sections3.
The Arcturus Engineering,
Inc. system (which manufactures Pix Cell IITM), bonds
the transfer film to the underneath of a vial cap placed on the surface
of the tissue. This allows the researcher to be able to see the tissue
section through the transparent transfer film when they are using the laser
pulse. (The size of the laser can be adjusted, but it is recommended
that it stay within 30 to 60 microns.) The cells can then be
transferred directly from the transfer film to a vial for molecular analysis.
The advantage of this procedure is that the desired cells retain their
morphologic features, to insure that the correct cells are being studied.
Computer software, developed by the NIH, stores images of the microdissected
cells both before and after LCM has been performed, and serves as a diagnostic
record. LCM can also be used to isolate DNA, RNA, and protein from
selected pure cells without damaging their macromolecules. The transfer
film is simply put inot a solution containing an enzyme buffer for DNA,
RNA, or protein. This buffer works to detach the desired product
away from the transfer film.The tissue section can be fixed in ethanol
or formalin, embedded in paraffin, or frozen. The tissue can also
be stained with a variety of stains to easily identify the desired cellsTM.
**Caution:
This is not a protocol or procedure for performing LCM!!! For more information
or a protocol, click here ***
For information on how to prepare tissue and slides for LCM click
here. 4
For some "helpful hints" about LCM click
here. 5