|
Erin Mckinley's Research
at Davidson
|
 |
From the common cold to HIV, we are all too familiar with the symptomatic effects of a viral infection. But in order to treat or prevent such infections, we have to learn more about the virus in general-its structure, replication mechanism, etc. Scientists have come a long way, but because so many different types of viruses exist, each with their own unique characteristics, we still have a long way to go. The virus we are working with is called reovirus. All of us have antibodies to protect ourselves against reovirus infection-that means that reovirus doesn't cause us to be sick or have some other negative reaction.So, you might wonder why we are even studying this virus if it poses us no threat. |
|
The answer is, that by learning more the reovirus we can learn more about viral pathogenesis in general. The information we learn from this model system could possibly be applied to vaccines (which are attenuated viruses) or other viruses that are harmful. The reovirus can be thought of as a little package of RNA wrapped in two concentric layers of protein. I've even compared it to a peanut M&M-you've got the important genetic code in the middle (the peanut) protected by an outer shell of protein (the chocolate). When the reovirus infects a cell, the outer shell breaks apart and falls of, allowing the virus to start replicating and taking over its host. The interactions between surface proteins in this outer shell determines the stability of the virus in environments between hosts. The stronger these interactions, the more resistant viruses may be to the many different chemical and physical agents that threaten viral propagation. |
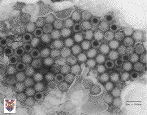 |
| An electron micrograph image of an animal reovirus. This image was used with permission from Queen's Univeristy at was obtained from www.virology.net. |
|
On the other hand, if these proteins bind to eachother too strongly and can't be removed from the outer shell (i.e. you can't bite through the chocolate to get to the peanut) then the virus won't be able to replicate. Thus, reoviruses face a trade-off between the stability of the outer shell necessary for survival between host cells and the "instability" of the outer shell essential for uncoating and replication within the host cell. A balance must be struck between the two opposing requirements (i.e. extracellular stability vs. intracellular "instability") representing a fundamental dilemma that must be solved by the virus. |
|
My experiment involves seven reovirus mutants that were isolated by my professor (Dr. David R. Wessner) as a grad student at Harvard. These mutants show an increased resistance to ethanol (an agent commonly used to disinfect). Our goal has been to determine whether these mutants are only ethanol resistant or are generally more stable, i.e. general stability mutants. To achieve this determination we have exposed the mutants to elevated temperature (which normally denature a protein and thus "kill" the virus) and phenol to see if they also show an increased resistance to these inactivating agents. The data from these experiments, in conjunction with past research, will help us better understand some of the interactions among proteins in the outer shell. |