Late (petechial) rash on palm and forearm
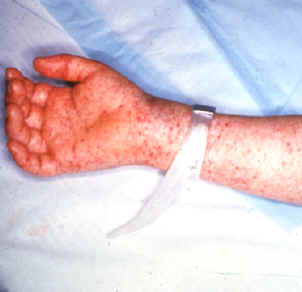
Photo courtesy of the CDC - http://www.cdc.gov/ncidod/dvrd/rmsf/Signs.htm
Although the rickettsial genomes are known, there has not been any virulence factors determined based on this information. The adhesins have not all been identified. In addition, another future study could be surrounding the gene for rickettsial phospholipase A2, which is hypothesized to be the mechanism by which the bacteria escape phagosomes upon initial infection. There is also a lack of evidence surrounding the in vitro vs. in vivo differences in the infection, especially due to the tick saliva modifications present in the infection of humans. Finally, there is also some confusion in the importance of the reactive oxygen species because this injury is not prevalent in animals. (Walker et al., 2003)
There is also a lot of room for research in the development of a vaccine for this disease. There are three vaccines against Rocky Mountain Spotted Fever, but none have proven to be sufficiently effective. These vaccines include the inactivation with Romalin in the infected tick tissue vaccine in the 1920s-30s, the infected chicken yolk sac vaccine withdrawn from use in the 1970s, and the infected cell culture vaccine that has never been released for public use. These vaccines all failed to adequately protect humans infected with R.rickettsii. (Williams et al., 1986) A new direction in the development of a vaccine is currently being explored surrounding the examination of the cross-protective immunity produced by the nonpathogenic strand R.rhipicephali to infections of R. rickettsii. If the protective antigens involved in both nonpathogenic and pathogen strands of rickettsiae can be identified and characterized, this could allow for the discovery of the mechanism of rickettsial immunity, which could then lead to a potential vaccine for the disease. (Gage et al., 1992)
The mechanism of rickettsial penetration into eukaryotic cells is still not fully understood. (Silverman et al., 1992) To learn more information about the possible areas of research in this field, as well as the current theories of this topic, see Pathogen Life Cycle.