Pathogen Life Cycle
General Description
Clostridium tetani is an anaerobic, rod-shaped bacterium that can be found in a variety of places, such as the soil and intestinal flora of domestic animals and humans (Farrar et al., 2000). C. tetani usually enter the body through an open wound, leading to spore germination under anaerobic conditions. This spore production gives the bacteria a drumstick-like appearance.
Once spore germination has occurred, toxins are released into the bloodstream and lymphatic system. These toxins act at several locations within the central nervous system, interfering with neurotransmitter release and blocking inhibitor impulses. Such disruptions lead to uncontrollable muscle contractions (Atkinson et al., 2006).
In the U.S., there are only 40 to 60 cases of tetanus infection per year. Globally, however, there are as many as 1 million deaths due to tetanus each year. Eighty percent of deaths due to tetanus infection occur in Africa and Asia due to poor vaccination procedures (Farrar et al., 2000).
Mechanism of Action
Tetanus toxin is composed of a heavy chain and light chain, which are attached by a disulfide bond (Figure 1). Tetanus toxin fragment C (TTFC) is a 47-kDa fragment on the heavy chain molecule that contains the ganglioside-binding domain (Robinson et al., 2003). TTFC attaches to gangliosides on the peripheral nerves, and as a result, the toxin is internalized. Through trans-synaptic spread, the toxin can spread to the central nervous system (Farrar et al., 2000).
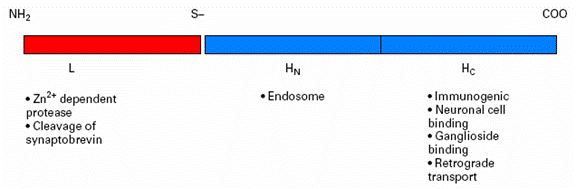 |
| Figure 1. Heavy (H) and light (L) chains of tetanus toxin, as well as their respective functions in the nervous system. Adapted from Farrar et al., 2000. |
The light chain contains a zinc metalloprotease domain which can cleave proteins that facilitate synaptic vessel fusion with the plasma membrane of the neuron – namely, the integral protein synaptobrevin. As a result, the neurotransmitter g-aminobutyric acid (GABA) is blocked from reaching the synaptic cleft, and the excitation of motor neurons persists. Persistent neuron signaling leads to the motor spasms seen in a typical tetanus patient (Farrar et al., 2000).
Phosphorylation of the Toxin
Neuron depolarization and the presence of extracellular calcium leads to tyrosine phosphorylation of the tetanus toxin light chain by Src kinase. Phosphorylation promotes a conformational change that folds the toxin into its active form; this folding drastically increases the catalytic activity of the toxin, as well as its thermal stability, leading to a more pronounced effect on the nervous system (Ferrer-Montiel et al., 1996).
This web page was produced as an assignment for an undergraduate course at
Davidson College.
Contact: cahermes@davidson.edu