More Students' Immunology Homepages
Immunology Homepage
Davidson Biology Homepage
This page was written by Christopher
Lee. Last updated 26 March 2000.
© Copyright 2000, Christopher Lee
by Christopher Lee
Adenosine Deaminase Deficiency
Return to my Immunology Homepage
Introduction Structure Functions Potential Binding Viruses Sources
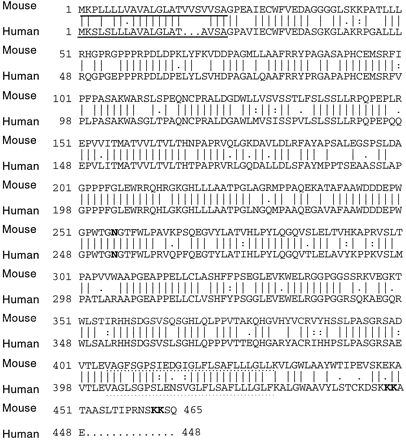
Figure 2: The compared sequences of human and mouse
tapasin proteins. 78% of the residues are identical. The solidly
underlined amino acids represent the N-terminal signal sequence, and the
dotted underlined sequence represent the C-terminal signal sequence.
The N-glycosylation site is in bold at aa#253. Figure reproduced
with permission from the author, Li et al., 1999.
Tapasin is normally found in stoichiometric amounts with TAP1/2 (Ortmann et al., 1997), which is expected under the predominate hypothesis that tapasin forms a complex with TAP1/2. Tapasin associates with the TAP1 protein of the TAP complex, and appears to bridge TAP and MHC I. (Humans MHC genes are also referred to as HLA, or human leukocite antigen.) TAP1, TAP2, and tapasin form a trimer, with calreticulin and MHC I more transiently associated (Li et al., 1999). It seems likely that TAP2 binds first with TAP1 to form a dimer, then the dimer associates with tapasin, which then associates with MHC I-calreticulin complex (Li et al., 1999) This sequence seems to imply that tapasin enjoys a broad role in the overall process of importing peptide fragments and loading them into MHC I molecules.
Functions Top Introduction Structure Potential Binding Viruses Sources
Besides physically joining MHC I and TAP complexes, tapasin appears to play a role in peptide loading of empty MHC molecules. There has also been evidence linking tapasin to efficient binding of peptide fragments to TAP. The presence of tapasin increases the concentration of TAP complexes in the ER (Lehner et al., 1999), magnifying the amount of peptides which can be introduced into the ER. The chaperone molecule of MHC I, calreticulin, associates only when the MHC I is empty, but the TAP1/2-tapasin trimer releases MHC I molecules when peptides are bound (Li et al., 1999). It is hypothesized that this mechanism allows tapasin to regulate MHC I delivery to the cell surface by retaining empty MHC I molecules in the ER until a peptide can bind.
Many of tapasin's functions has been discovered through the 721.220 human mutant line, in which MHC I complexes are not displayed on the surface of the protein, and tapasin is not expressed (Ortmann et al., 1997). TAP1/2 are normally expressed, and peptide fragments are translocated into the lumen of the ER (Li et al., 1999). The malfunction lies in the inability of MHC I to associate with the TAP complex. In fact, the MHC I dimer appears to lack any associated proteins (Li, et al., 1999). Transfection of tapasin into 721.220 cells results in revitalization of MHC I-TAP complexes, and MHC I-peptide bonding. In two MHC alleles (HLA.A1 and HLA.B8), this tapasin transfection increased surface MHC I presence ten-fold (Ortmann et al., 1997). Other alleles also show increased MHC expression (Lavau et al., 1999). This strongly implicates tapasin as the bridge between TAP and MHC I, and similar results in a study of b2-m-deficient Daudi cells further indicate that tapasin binds to the MHC I-alpha, or -heavy, chain (Li et al., 1997).
A soluble version of tapasin (without the membranal region) associates with MHC I but not with TAP. The transfection of soluble tapasin into the .220 cell line results in restored surface expression of MHC I molecules (Lehner et al., 1999), but peptide translocation remains deficient. This is explained by the suspected function of tapasin to regulate release of MHC I from the ER to the Golgi complex (Schoenhals et al., 1999). When membranal tapasin is not expressed, this regulation is lost, and MHC is released to the surface more readily (Li et al., 2000).
Potential Binding Viruses Top Introduction Structure Functions Sources
There are a number of viruses which inhibit TAP transport of peptides by binding to the TAP complex, preventing peptide translocation from the cytosol to the ER, thereby preventing the host from recognizing viral activity in the cell. The herpes simplex virus is one such virus, but experiments involving normal HLA alleles, and the 721.220 mutant result in virtually idential inhibition of TAP transport, indicating that tapasin is not directly attacked by the virus (Lacaille and Androlewicz, 1998). The human cytomegalovirus US6 gene encodes a 22 kDa protein which also binds the TAP complex, and there has been some speculation that this protein could bind to one of the TAP subunits, or possibly to tapasin (Lehner et al., 1997).
Grandea III AG, Androlewicz MJ, Athwal RS, Geraghty DE, Spies T. 1995 October. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 270: 105-108.
Lacaille VG, Androlewicz MJ. 1998 July 10. Herpes simplex virus inhibitor ICP47 destabilizes the transporter associated with antigen processing (TAP) heterodimer. Journal of Biological Chemistry. 273: 17386-17390. Abstract and Full Text
Lauvau G, Gubler B, Cohen H, Daniel S, Caillat-Zucman S, van Endert PM. 1999 October. Tapasin enhances assembly of transporters associated with antigen processing-dependednt and -independent peptides with HLA-A2 and HLA-B27 expressed in insect cells. The Journal of Biological Chemistry. 274:31349-58. Abstract and Full Text
Lehner PJ, Surman MJ, Cresswell P. 1998 Feb. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 8(2):221-31 Medline Abstract
Lehner PJ, Karttunen JT, Wilkinson GWG, Cresswell P. 1997 June. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proceedings of the National Acadademy of Science. 94: 6904-6909. Abstract and Full Text
Li S, Paulsson KM, Sjögren H, Wang P. 1999 March. Peptide-bound major histocompatibility complex class I molecules associate with tapasin before dissociation from transporter associated with antigen processing. The Journal of Biological Chemistry. 274:8649-54. Abstract and Full Text
Li S, Paulsson KM, Chen S, Sjögren H, Wang P. 2000 January. Tapasin is required for efficient peptide binding to transporter associated with antigen proceesing. The Journal of Biological Chemistry. 275:1581-86. Abstract Full Text (requires subscription or article purchase)
Li S, Sjögren H, Hellman U, Pettersson RF, Wang P. 1997 August. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proceedings of the National Acadademy of Science. 94: 8708-8713. Full Text
Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. 1997 August. A critical role for tapasin in the assembly and function of multimerica MHC class I-TAP complexes. Science. 277:1306-9.
Schoenhals GJ, Krishna RM, Grandea III AG, Spies T, Peterson PA, Yang Y, Früh K. 1999. Retention of empty MHC class I molecules by tapasin is essential to reconstitute antigen presentation in invertebrate cells. The European Molecular Biology Organization Journal. 18:743-753. Abstract
Relevant link:
Peter
Cresswell, Yale University
This page was written by Christopher
Lee. Last updated 26 March 2000.
© Copyright 2000, Christopher Lee