Borrelia burgdorferi
Innate Immune Response
After B. burgdorferi is injected into the host via tick bite, it typically remains localized in the skin, causing the erythema migrans rash. Fever, mononuclear cell response, and flu-like symptoms may occur in conjunction with the rash, but specific antibody response is lacking until the bacterium spreads into the host’s bloodstream (Steere, 1989). Differences in bacterial surface proteins (Osp) have been shown to induce differential host responses, and only certain surface proteins are able to induce disseminated infection (Steere, 1989).
Upon entering the body, B. burgdorferi initiates the innate immune response. Monocytes, granulocytes, and dendritic cells are first called to the site of infection near the tick bite to begin phagocytosis. Dendritic cells are likely the most important cell in the first line of defense against B. burgdorferi once it has entered the skin. Dendritic cells then internalize the spirochete (Filguera et. al., 1996). These immune cells use traditional phagocytosis, coiling phagocytosis, or tube phagocytosis. In coiling phagocytosis, seen in monocytes, bacteria attach to the surface of the phagocyte and get rolled into a single fold of the plasma membrane, forming a pseudopod coil. The phagocyte forms multiple folds of the plasma membrane around the bacterium, which eventually leads to engulfment of the bacterium. Tube phagocytosis, used by neutrophils, is induced when the bacterium attaches to the neutrophil surface. The neutrophil plasma membrane creates a tube-like protrusion to surround the bacterium, and when this tube has covered the entire bacterium, the protrusion is drawn back into the neutrophil cell body, bringing the bacterium with it (Diterich and Hartung, 2001).
Once phagocytosed, the bacteria are destroyed using lysosomes or other means. Non-lysosomal bacterial destruction involves the production of toxic substances such as nitric oxide and oxygen radicals, as well as the complement-induced killing processes including oxidative burst and calcium mobilization (Diterich and Hartung, 2001). Both opsonized and unopsonized B. burgdorferi have been shown to induce respiratory or oxidative burst after exposure to phagocytic cells such as PMNs (Szczepanski and Benach, 1991). Studies show that the Borrelia spirochete does not typically survive within the phagocytic cell, which is demonstrated by the presence of bacterial debris and nonviable bacteria within phagocytes (Szczepanski and Benach, 1991).
Summary of host immune response to B. burgdorferi infection
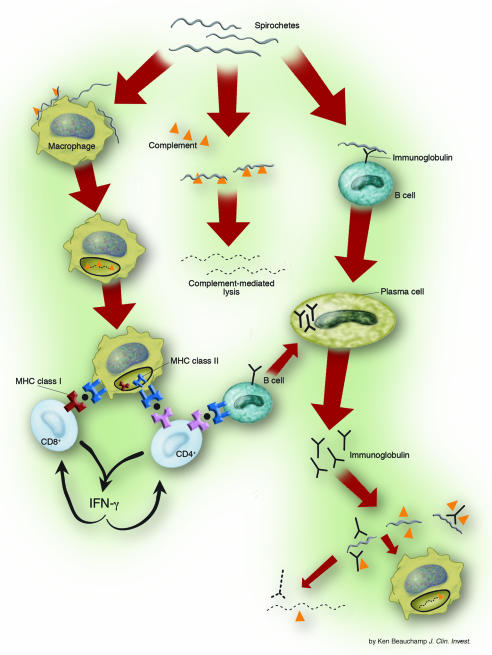
Image courtesy of [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=385417]
An important non-specific response induced by B. burgdorferi is the activation of the complement system. Complement activation produces C5a, which aids in pathogen opsonization and the subsequent attraction of macrophages and polymorphonuclear leukocytes (PMN) to phagocytose the invading microbe (Szczepanski and Benach, 1991). Opsonization also allows Fc receptor-mediated uptake by PMN and monocytes. Studies have shown that un-opsonized bacteria are taken up by phagocytic cells less often than those that are opsonized. In addition, down-regulation of the Fc receptor led to a 90% decrease in bacterial uptake, demonstrating the importance of these receptors in phagocytic events during the innate immune response against B. burgdorferi (Szczepanski and Benach, 1991).
PAMPs (Pathogen associated molecular proteins) are molecules commonly found in pathogens that bind to toll-like receptors (TLR) and induce the production of cytokines. A key aspect of the innate immune response to B. burgdorferi is induced when bacterial lipoproteins bind to CD14 and TLR-2 on macrophages – an interaction that leads to the production of inflammatory cytokines (Steere, 2001). The surface of B. burgdorferi contains a lipopolysaccharide-like (LPS) molecule, which has properties similar to an endotoxin. This molecule is a lipooligosaccharide moiety, and is most likely the PAMP which activates TLRs in the innate immune response (Szczepanski and Benach, 1991).
Dendritic cells form a vital link between the innate and adaptive immune response and are important in phagocytosing B. burgdorferi and secreting cytokines IL-8, IL-12, TNF-α, and IL-1. Mice that are unable to produce TNF-α show increased susceptibility to B. burgdorferi, while humans with high levels of TNF-α in the CSF are less likely to develop chronic Lyme disease. A study by Sjöwall et. al demonstrated the importance of TNF-α in effective early innate immune response. In this study, subjects with higher levels of TNF-α were more likely to be asymptomatic, thereby demonstrating a more effective immune response (Sjöwall et. al., 2005).
This page was created for an undergraduate Immunology course, Biology 307, at Davidson College in the Spring semester of 2007 under Dr. Sophia Sarafova (sasarafova@davidson.edu)
Please direct all comments and questions to Meredith Prasse (meprasse@davidson.edu)