This website was produced as an assignment for an undergratuate course at Davidson College
Home safsaProtein dsafOrthologssdaf Jmol Structurefdas Review Paper

What Is an Ortholog and Why Are They Important?
Orthologs are genes that have evolved from a common ancestor gene. These genes can be in different species or within the same species. Othologs can be identified by comparing either gene or amino acid sequences and looking for similarity. Learn more about building genetic trees at The History of Life. BLAST protein searches can scan all the proteins of known sequence and look for correspondence with your protein of interest.
Orthologs can help us in several ways. First by examining minor differences between orthologous genes in different species we can create evolutionary trees. An example of an evolutionary tree can be seen below in Figure 1. Greater similarity indicates that those species are more closely related because they share a closer common ancestor. Second sequence similarity can be related to functional similarity. Orthologous proteins may perform similar purposes, therefore proteins with known functions can help characterize those with unknown functions.
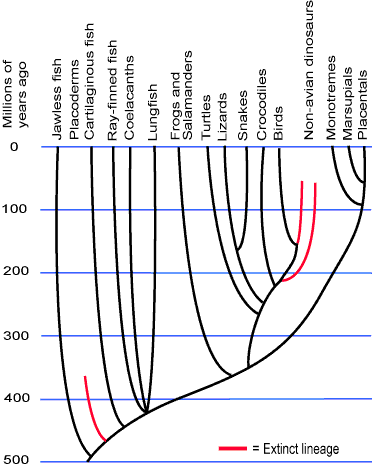
Image From: Evolution 101: Adding Time
Figure 1: An example of a genetic tree, just of vertibrates. Millions of years are respresented by the vertical axes. The further species are away from one another on the horizontal axis, the less close their common ancestor is.
Are Orthologs to PheOH Found In Other Species?
Phenylalanine Hydroxylase (PheOH) can be found in numerous other species including Pan troglodytes (chimpanzee), Canis lupus familiaris (dog), Bos taurus (bull), Mus musculus (mouse) , Rattus norvegicus (rat), Gallus gallus (chicken), Danio rerio (zebrafish), Drosophila melanogaster (fruitfly), Anopheles gambiae (mosquito), and Caenorhabditis elegans (flatworm). Clicking on each of the different species will show you a blast comparison of their PheOH with human PheOH. Many of these species share greater than 90% of the protein sequence, indicating that the majority of the protein is highly conserved. Figure below shows the percent of amino acid and nucleotide sequence similarity between human PheOH and a variety of other species.

Image From: HomoloGene
Figure 2: Table showing the relative percent similarity between PheOH in multiple species and human PheOH.
Looking through the BLAST comparisons of the various species' PheOH proteins compared to human PheOH can tell us several things about the protein. In general the amino acids from approximately 100 to 350 are the most conserved. This observation fits with our understanding of how PheOH works, and the domains it contains. Given that the amino acid phneylalanine is not species specific, the catalytic site, approxiametely amino acids 115 to 415, should be remarkably similar across species. This proves to be true, even in C. Elegans, the most different of the orthologs. Differences at the beginning and end of the protein are due to conformational and regulatory differences. Although in humans PheOH functions as a tetrameric unit, occasionally only two of the subunits can be found together. These PheOH dimers are found more frequently in other species. Therefore the tetramerization area of the protein would be different, the optimal amino acid sequence for the joining of four subunits may not be the same as the optimal sequence for a dimer.
Along the same lines PheOH can be regulated in different manners. In humans PheOH is regulated by Phe, tetrahydrobiopterin, and phosphorylation. However in non vertebrate species such as Danio rerio, Drosophila, and the rest of the least similar species, PheOH isn't primarily found in the liver, mostly because they don't have livers. Therefore Phe is never amassed in one location like in humans. Without high concentrations on Phe, PheOH cannot be substrate regulated. Shiman et al. (1982) found that high levels of Phe need to exist before PheOH increases by a measurable amount. Therefore other methods of regulation, such as phosporylation and tetrahydrobiopterin become more important. With different regulation comes different structure and consequently different amino acids.
Are There Other Human Proteins Similar to PheOH?
The proteins Tryptophan Hydroxylase (TrpOH) and Tyrosine Hydroxylase (TyrOH) are orthologs of PheOH. All three proteins add –OH groups to the carbon rings on their respective amino acids; together they form the small family of enzymes called aromatic amino acid hydroxylases. TrpOH adds an –OH group to the 5’ carbon of Tryptophan, and is the first step in the synthesis of serotonin. TyrOH adds an –OH group to the 3’ carbon of tyrosine and is the first step in the synthesis of norepinephrine.
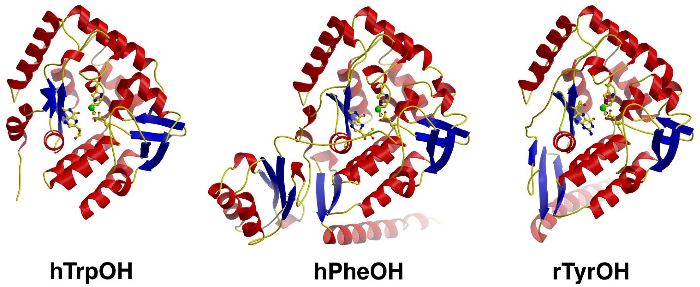
Image From: The Stevens Laboratory
Figure 3: Diagrams of TrpOH, PheOH, and TyrOH.
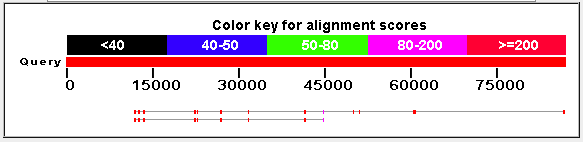
Image From: BLAST
Figure 4: Alignment of the nucleotide sequences of TrpOH (top) and TyrOH (bottom with PheOH. The higher the alignment score, the more similar that area of the protein is.
Generally speaking nucleotide sequences are more dissimilar than amino acid sequences. In comparison to the BLAST results above, when using the nucleotide sequences, the amino acid sequences are considerably more similar. This is due to a couple of reasons. First mutliple codons can code for the same amino acid, so nueclotides can be different while amino acids are the same. Second, like most proteins, the gene sequence is made up of introns and exons. Changes in the nucelotide sequence within introns would not affect the ultimate amino acid sequence. PheOh has 452 amino acids, while TrpOH has 490, and TyrOh has 497. Figures below shows a BLAST alignment of each protein with PheOH. Both expected values are infintesimal, nearly zero, indicating a high probability that these proteins are related and not randomly similar. 52% of the amino acids in TyrOH match PheOH and 54% of the amino aicds in TrpOH match PheOH.

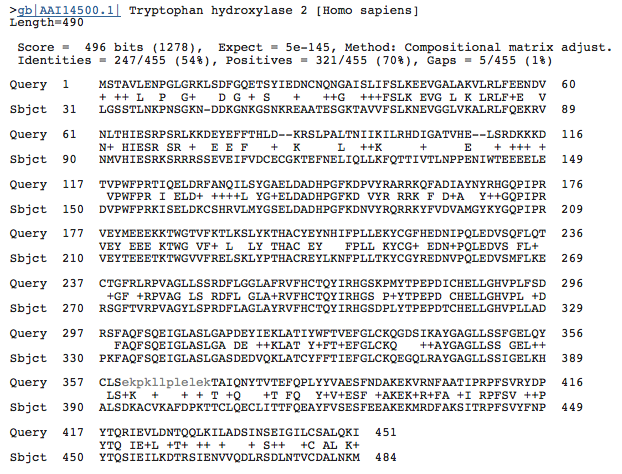
Image From: BLAST
Figures 5 and 6: BLAST results comparing the amino acid sequence of TyrOH and PheOH (left) and TrpOH and PheOH (right). The low expected values for both indicate the similarities are not random chance.
References:
Dutta, Shuchismita and David S. Goodsell. “Phenylalanine Hydroxylase.” Molecule of the Month. January 2005. Protein Data Bank. Accessed 8 March 2010.
Fusetti F, Erlandsen H, Flatmark T, Stevens R. 1998 March. Structure of Tetrameric Human Phenylalanine Hydroxylase and Its Implications for Phenylketonuria. Journal of Biological Chemistry 273: 16962-16967.
McDowell, Jennifer. "Phenylalanine Hydroxylase." Protein of the Month. January 2005. European Bioinformatics Institute. Accessed 8 March 2010.
Shiman R, et al. 1982 July.Regulation of Phenylalanine Hydroxylase Activity by Phenylalanine in Vivo, in Vitro, and in Perfused Rat Liver. The Journal of Biological Chemistry 257: 11213-11216.
Email me: capiper@davidson.edu