Bordetella pertussis is the causative agent of the disease pertussis (Whooping cough). Whooping cough is most commonly seen in infants, where the vocal inspiration between spells of paroxysmal coughing gives the disease its name (Bjornstad and Harvill 2005). Bordetella pertussis is a small gram-negative coccobacilli that is non-motile and pathogenic only to humans (Hoppe 1999). The bacteria are observed in culture as single or paired entities which grow slowly and fastidiously (Todar 2004).
Pertussis has a number of different stages. The first stage is the catarrhal or colonization phase. Here, symptoms resemble a cold or an upper respiratory infection, but remain relatively nonspecific. Over the first ten days of infection, coughing, fever, and malaise may be observed (Todar 2004). The toxemic, or paroxysmal, stage is characterized by severe, prolonged coughing. This coughing is accompanied by the inspiratory “whoop” at this stage (Todar 2004, Wood and Friedman 1998). The final, convalescent phase is marked by reduction in attacks of severe coughing over three to four weeks (Wood and Friedman 1998).
Bordetella pertussis can only live independently inside a human host. It infects the upper airway (the throat and nose), as well as the lungs (Jenkinson 2005). Thus, the life cycle of the bacteria must occur within these structures. B. pertussis is easily killed in humans, whether by antibiotics or natural immune responses (Jenkinson 2005). So, the bacteria depend upon a high rate of transmission to continue survival. Recent statistics reveal that for each independent infection with B. pertussis, fifteen secondary infections occur (Bjornstad and Harvill 2005). Transmission of the bacteria is via airborne droplets produced by coughing (Bjornstad and Harvill 2005).
Like other bacteria, B. pertussis can divide by binary fission. The growth of a population of these bacteria is slow in culture. B. pertussis uses amino acids as an energy source (Wood and Friedman 1998). The specific resource demands (it cannot be grown on normal media, but rather must be supplied with Mueller-Hinton agar augmented with five percent horse blood) and sensitivity to cold and desiccation challenge this bacteria’s survival in a single host (Hoppe 1999). Thus, many of the disease pathologies linked to B. pertussis are a result of its many secreted toxins and adherence factors.
In the first stage of colonization, the bacteria attach to ciliated airway epithelium. Filamentous hemagglutinin (FHA), fimbrae, and pertactin (PRN) help mediate adherence to epithelium. FHA is a cell surface structure that binds galactose on sulfatide (a sulfated glycolipid on ciliated epithelium cells). Pertussis toxin (PT) is also a major adhesin. This six-subunit structure is both secreted and found on the cell surface. The S2 subunit can bind lactosylceramide (a glycolipid on ciliated epithelium cells) while the S3 subunit can bind a phagocytic cell surface glycoprotein (Todar 2004). Tracheal cytotoxin (TCT) also plays an important role in colonization by damaging ciliated epithelium cells, decreasing the infected individual’s natural ability to clear the bacteria from airways (Todar 2004).
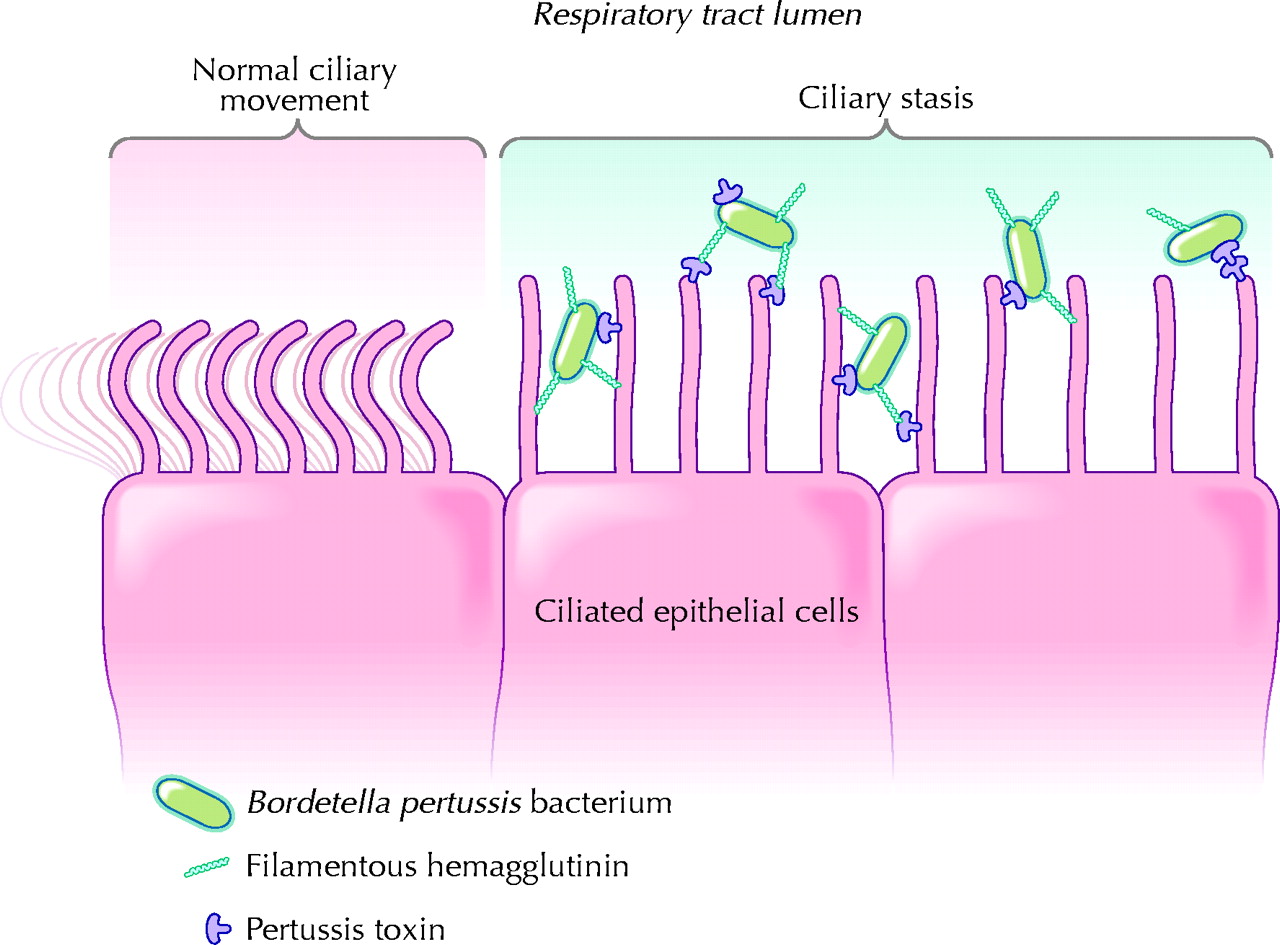 image: Tozzi et al. 2005
image: Tozzi et al. 2005
After colonization, the bacteria may divide and continue to produce toxins and adhesins. The original organisms die, are killed by an immune response or an antibiotic, or are transmitted to another human, where they will colonize the airways, produce toxin and adhesins, and transmit again. It is important to note that B. pertussis is known to possess mechanisms to bind to cells as well as enter into them. B. pertussis may enter and spend a small part of its life cycle in epithelial cells, monocytes, and neutrophils (Fedele et al. 2005). While the bacteria show low survival rates in dendritic cells, the possible role of intracellular pathogenesis in pertussis is still debated (Todar 2004).